The application of Bonelike® Poro as a synthetic bone substitute for the management of critical-sized bone defects - A comparative approach to the autograft technique - A preliminary study
- PMID: 33981810
- PMCID: PMC8082556
- DOI: 10.1016/j.bonr.2021.101064
The application of Bonelike® Poro as a synthetic bone substitute for the management of critical-sized bone defects - A comparative approach to the autograft technique - A preliminary study
Abstract
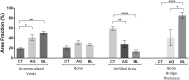
The effective treatment of non-unions and critical-sized defects remains a challenge in the orthopedic field. From a tissue engineering perspective, this issue can be addressed through the application bioactive matrixes to support bone regeneration, such as Bonelike®, as opposed to the widespread autologous grafting technique. An improved formulation of Bonelike® Poro, was assessed as a synthetic bone substitute in an ovine model for critical-sized bone defects. Bone regeneration was assessed after 5 months of recovery through macro and microscopic analysis of the healing features of the defect sites. Both the application of natural bone graft or Bonelike® Poro resulted in bridging of the defects margins. Untreated defect remained as fibrous non-unions at the end of the study period. The characteristics of the newly formed bone and its integration with the host tissue were assessed through histomorphometric and histological analysis, which demonstrated Bonelike® Poro to result in improved healing of the defects. The group treated with synthetic biomaterial presented bone bridges of increased thickness and bone features that more closely resembled the native spongeous and cortical bone. The application of Bonelike® Poro enabled the regeneration of critical-sized lesions and performed comparably to the autograph technique, validating its octeoconductive and osteointegrative potential for clinical application as a therapeutic strategy in human and veterinary orthopedics.
Keywords: Bone regeneration; Bonelike®; Ceramic biomaterials; Critical bone defects; Ovine model.
© 2021 Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Alkhraisat M.H., Rueda C., Jerez L.B., Mariño F.T., Torres J., Gbureck U. Effect of silica gel on the cohesion, properties and biological performance of brushite cement. Acta Biomater. 2010;6(1):257–265. - PubMed
-
- Anderson ML, Dhert WJ, de Bruijn JD, Dalmeijer RA, Leenders H, van Blitterswijk CA, et al. Critical size defect in the goat's os ilium: A model to evaluate bone grafts and substitutes. Clinical Orthopaedics and Related Research®. 1999;364:231–9. - PubMed
-
- Atayde L., Cortez P., Pereira T., Armada-da-Silva P., Afonso A., Lopes M. A new sheep model with automatized analysis of biomaterial-induced bone tissue regeneration. J. Mater. Sci. Mater. Med. 2014;25(8):1885–1901. - PubMed
-
- Atayde L., Cortez P., Afonso A., Santos M., Maurício A., Santos J. Morphology effect of bioglass-reinforced hydroxyapatite (Bonelike®) on osteoregeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103(2):292–304. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

