Reciprocal interactions between osteoclasts and nociceptive sensory neurons in bone cancer pain
- PMID: 33981921
- PMCID: PMC8108580
- DOI: 10.1097/PR9.0000000000000867
Reciprocal interactions between osteoclasts and nociceptive sensory neurons in bone cancer pain
Abstract
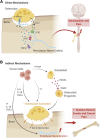
Many common cancers such as breast, prostate, and lung cancer metastasize to bones at advanced stages, producing severe pain and functional impairment. At present, the current pharmacotherapies available for bone cancer pain are insufficient to provide safe and efficacious pain relief. In this narrative review, we discuss the mechanisms used by cancer cells within the bone tumor microenvironment (TME) to drive bone cancer pain. In particular, we highlight the reciprocal interactions between tumor cells, bone-resorbing osteoclasts, and pain-sensing sensory neurons (nociceptors), which drive bone cancer pain. We discuss how tumor cells present within the bone TME accelerate osteoclast differentiation (osteoclastogenesis) and alter osteoclast activity and function. Furthermore, we highlight how this perturbed state of osteoclast overactivation contributes to bone cancer pain through (1) direct mechanisms, through their production of pronociceptive factors that act directly on sensory afferents; and (2) by indirect mechanisms, wherein osteoclasts drive bone resorption that weakens tumor-bearing bones and predisposes them to skeletal-related events, thereby driving bone cancer pain and functional impairment. Finally, we discuss some potential therapeutic agents, such as denosumab, bisphosphonates, and nivolumab, and discuss their respective effects on bone cancer pain, osteoclast overactivation, and tumor growth within the bone TME.
Keywords: Nociceptor; bone cancer; cancer pain; neuroimmune interactions; osteoclast.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The International Association for the Study of Pain.
Conflict of interest statement
R.R. Ji is a consultant of Boston Scientific and received research support from the company. These activities are not related to this review. The remaining authors have no conflicts of interest to disclose. C.R. Donnelly received support from the John J. Bonica Trainee Fellowship and NIH T32 GM008600. Illustrations were created by A.S. Andriessen and C.R. Donnelly using BioRender.Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures
Similar articles
-
Mechanisms of bone metastasis.Cancer. 1997 Oct 15;80(8 Suppl):1546-56. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1546::aid-cncr4>3.3.co;2-r. Cancer. 1997. PMID: 9362421 Review.
-
Acidic microenvironment and bone pain in cancer-colonized bone.Bonekey Rep. 2015 May 6;4:690. doi: 10.1038/bonekey.2015.58. eCollection 2015. Bonekey Rep. 2015. PMID: 25987988 Free PMC article. Review.
-
Heterogeneity of tumor cells in the bone microenvironment: Mechanisms and therapeutic targets for bone metastasis of prostate or breast cancer.Adv Drug Deliv Rev. 2016 Apr 1;99(Pt B):206-211. doi: 10.1016/j.addr.2015.11.017. Epub 2015 Dec 4. Adv Drug Deliv Rev. 2016. PMID: 26656603 Review.
-
The role of osteoclastic activity in prostate cancer skeletal metastases.Drugs Today (Barc). 2002 Feb;38(2):91-102. doi: 10.1358/dot.2002.38.2.820105. Drugs Today (Barc). 2002. PMID: 12532187 Review.
-
Osteoprotegerin diminishes advanced bone cancer pain.Cancer Res. 2001 May 15;61(10):4038-47. Cancer Res. 2001. PMID: 11358823
Cited by
-
Glycogen synthase kinase-3β inhibition decreases inflammation and relieves cancer induced bone pain via reducing Drp1-mediated mitochondrial damage.J Cell Mol Med. 2022 Jul;26(14):3965-3976. doi: 10.1111/jcmm.17432. Epub 2022 Jun 10. J Cell Mol Med. 2022. PMID: 35689386 Free PMC article.
-
Introduction for special issue on neuroimmune interactions in chronic pain.Pain Rep. 2021 Mar 9;6(1):e894. doi: 10.1097/PR9.0000000000000894. eCollection 2021. Pain Rep. 2021. PMID: 33981928 Free PMC article. Review.
-
GPR37 Activation Alleviates Bone Cancer Pain via the Inhibition of Osteoclastogenesis and Neuronal Hyperexcitability.Adv Sci (Weinh). 2025 Apr;12(14):e2417367. doi: 10.1002/advs.202417367. Epub 2025 Feb 18. Adv Sci (Weinh). 2025. PMID: 39965073 Free PMC article.
-
A metastasis-on-a-chip approach to explore the sympathetic modulation of breast cancer bone metastasis.Mater Today Bio. 2022 Feb 14;13:100219. doi: 10.1016/j.mtbio.2022.100219. eCollection 2022 Jan. Mater Today Bio. 2022. PMID: 35243294 Free PMC article.
-
Nociceptor mechanisms underlying pain and bone remodeling via orthodontic forces: toward no pain, big gain.Front Pain Res (Lausanne). 2024 Feb 22;5:1365194. doi: 10.3389/fpain.2024.1365194. eCollection 2024. Front Pain Res (Lausanne). 2024. PMID: 38455874 Free PMC article. Review.
References
-
- Alam AS, Gallagher A, Shankar V, Ghatei MA, Datta HK, Huang CL, Moonga BS, Chambers TJ, Bloom SR, Zaidi M. Endothelin inhibits osteoclastic bone resorption by a direct effect on cell motility: implications for the vascular control of bone resorption. Endocrinology 1992;130:3617–24. - PubMed
-
- Awolaran O, Brooks SA, Lavender V. Breast cancer osteomimicry and its role in bone specific metastasis; an integrative, systematic review of preclinical evidence. Breast 2016;30:156–71. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
