Time-Restricted Feeding Regulates Circadian Rhythm of Murine Uterine Clock
- PMID: 33981944
- PMCID: PMC8099714
- DOI: 10.1093/cdn/nzab064
Time-Restricted Feeding Regulates Circadian Rhythm of Murine Uterine Clock
Abstract
Background: Skipping breakfast is associated with dysmenorrhea in young women. This suggests that the delay of food intake in the active phase impairs uterine functions by interfering with circadian rhythms.
Objectives: To examine the relation between the delay of feeding and uterine circadian rhythms, we investigated the effects of the first meal occasion in the active phase on the uterine clock.
Methods: Zeitgeber time (ZT) was defined as ZT0 (08:45) with lights on and ZT12 (20:45) with lights off. Young female mice (8 wk of age) were divided into 3 groups: group I (ad libitum consumption), group II (time-restricted feeding during ZT12-16, initial 4 h of the active period), and group III (time-restricted feeding during ZT20-24, last 4 h of the active period, a breakfast-skipping model). After 2 wk of dietary restriction, mice in each group were killed at 4-h intervals and the expression profiles of uterine clock genes, Bmal1 (brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1), Per1 (period circadian clock 1), Per2, and Cry1 (cryptochrome 1), were examined.
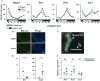
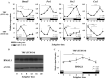
Results: qPCR and western blot analyses demonstrated synchronized circadian clock gene expression within the uterus. Immunohistochemical analysis confirmed that BMAL1 protein expression was synchronized among the endometrium and myometrium. In groups I and II, mRNA expression of Bmal1 was elevated after ZT12 at the start of the active phase. In contrast, Bmal1 expression was elevated just after ZT20 in group III, showing that the uterine clock rhythm had shifted 8 h backward. The changes in BMAL1 protein expression were confirmed by western blot analysis.
Conclusions: This study is the first to indicate that time-restricted feeding regulates a circadian rhythm of the uterine clock that is synchronized throughout the uterine body. These findings suggest that the uterine clock system is a new candidate to explain the etiology of breakfast skipping-induced uterine dysfunction.
Keywords: circadian rhythms; clock gene; dysmenorrhea; meal timing; uterine function.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Society for Nutrition.
Figures


References
-
- Fujiwara T. Skipping breakfast is associated with dysmenorrhea in young women in Japan. Int J Food Sci Nutr. 2003;54(6):505–9. - PubMed
-
- Angelin P, Dileep D, Manju T, Veena M, Pradeep D, Amreen K, Soumitra S. Effect of skipping breakfast on young girls’ menstruation. Indian J Youth Adolesc Health. 2017;4:17–20.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

