Relation of Quantitative Histologic and Radiologic Breast Tissue Composition Metrics With Invasive Breast Cancer Risk
- PMID: 33981950
- PMCID: PMC8103888
- DOI: 10.1093/jncics/pkab015
Relation of Quantitative Histologic and Radiologic Breast Tissue Composition Metrics With Invasive Breast Cancer Risk
Abstract
Background: Benign breast disease (BBD) is a strong breast cancer risk factor, but identifying patients that might develop invasive breast cancer remains a challenge.
Methods: By applying machine-learning to digitized hematoxylin and eosin-stained biopsies and computer-assisted thresholding to mammograms obtained circa BBD diagnosis, we generated quantitative tissue composition metrics and determined their association with future invasive breast cancer diagnosis. Archival breast biopsies and mammograms were obtained for women (18-86 years of age) in a case-control study, nested within a cohort of 15 395 BBD patients from Kaiser Permanente Northwest (1970-2012), followed through mid-2015. Patients who developed incident invasive breast cancer (ie, cases; n = 514) and those who did not (ie, controls; n = 514) were matched on BBD diagnosis age and plan membership duration. All statistical tests were 2-sided.
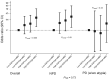
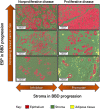
Results: Increasing epithelial area on the BBD biopsy was associated with increasing breast cancer risk (odds ratio [OR]Q4 vs Q1 = 1.85, 95% confidence interval [CI] = 1.13 to 3.04; P trend = .02). Conversely, increasing stroma was associated with decreased risk in nonproliferative, but not proliferative, BBD (P heterogeneity = .002). Increasing epithelium-to-stroma proportion (ORQ4 vs Q1 = 2.06, 95% CI =1.28 to 3.33; P trend = .002) and percent mammographic density (MBD) (ORQ4 vs Q1 = 2.20, 95% CI = 1.20 to 4.03; P trend = .01) were independently and strongly predictive of increased breast cancer risk. In combination, women with high epithelium-to-stroma proportion and high MBD had substantially higher risk than those with low epithelium-to-stroma proportion and low MBD (OR = 2.27, 95% CI = 1.27 to 4.06; P trend = .005), particularly among women with nonproliferative (P trend = .01) vs proliferative (P trend = .33) BBD.
Conclusion: Among BBD patients, increasing epithelium-to-stroma proportion on BBD biopsies and percent MBD at BBD diagnosis were independently and jointly associated with increasing breast cancer risk. These findings were particularly striking for women with nonproliferative disease (comprising approximately 70% of all BBD patients), for whom relevant predictive biomarkers are lacking.
Published by Oxford University Press 2021.
Figures


References
-
- Silverstein M. Where’s the outrage? J Am Coll Surg. 2009;208(1):78–79. - PubMed
-
- Gutwein LG, Ang DN, Liu H, et al.Utilization of minimally invasive breast biopsy for the evaluation of suspicious breast lesions. Am J Surg. 2011;202(2):127–132. - PubMed
-
- Dupont WD, Page DL.. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146–151. - PubMed
-
- Hartmann LC, Sellers TA, Frost MH, et al.Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353(3):229–237. - PubMed
-
- Santen RJ, Mansel R.. Benign breast disorders. N Engl J Med. 2005;353(3):275–285. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
