EEG alterations during wake and sleep in mild cognitive impairment and Alzheimer's disease
- PMID: 33981973
- PMCID: PMC8086022
- DOI: 10.1016/j.isci.2021.102386
EEG alterations during wake and sleep in mild cognitive impairment and Alzheimer's disease
Abstract
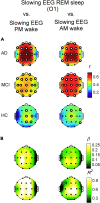
Patients with Alzheimer's disease (AD) undergo a slowing of waking electroencephalographic (EEG) rhythms since prodromal stages, which could be ascribed to poor sleep quality. We examined the relationship between wake and sleep alterations by assessing EEG activity during sleep and (pre-sleep/post-sleep) wakefulness in AD, mild cognitive impairment (MCI) and healthy controls. AD and MCI show high sleep latency and less slow-wave sleep. Reduced sigma activity characterizes non-rapid eye movement (NREM) sleep, reflecting sleep spindles loss. The EEG slowing characterizes REM sleep and wakefulness of AD and MCI, with strong correlations among the two phenomena suggesting common neuropathological mechanisms. Evening-to-morning variations in waking EEG revealed the gradual disappearance in MCI and AD of overnight changes in delta activity, indicating a progressive decay of sleep restorative functions on diurnal activity that correlates with the impairment of sleep high-frequency activity in AD. Our findings support a linkage between wake and sleep alterations, and the importance of sleep-related processes in Alzheimer's disease progression.
Keywords: Chronobiology; Cognitive Neuroscience; Human Physiology.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Babiloni C., Binetti G., Cassetta E., Forno G.D., Percio C. Del, Ferreri F., Ferri R., Frisoni G., Hirata K., Lanuzza B. Sources of cortical rhythms change as a function of cognitive impairment in pathological aging: a multicenter study. Clin. Neurophysiol. 2006;117:252–268. doi: 10.1016/j.clinph.2005.09.019. - DOI - PubMed
-
- Babiloni C., Lizio R., Del Percio C., Marzano N., Soricelli A., Salvatore E., Ferri R., Cosentino F.I.I., Tedeschi G., Montella P. Cortical sources of resting state EEG rhythms are sensitive to the progression of early stage Alzheimer’s disease. J. Alzheimer’s Dis. 2013;34:1015–1035. doi: 10.3233/JAD-121750. - DOI - PubMed
-
- Babiloni C., Lizio R., Marzano N., Capotosto P., Soricelli A., Triggiani A.I., Cordone S., Gesualdo L., Del Percio C. Brain neural synchronization and functional coupling in Alzheimer’s disease as revealed by resting state EEG rhythms. Int. J. Psychophysiol. 2015;103:88–102. doi: 10.1016/j.ijpsycho.2015.02.008. - DOI - PubMed
-
- Babiloni C., Triggiani A.I., Lizio R., Cordone S., Tattoli G., Bevilacqua V., Soricelli A., Ferri R., Nobili F., Gesualdo L. Classification of single normal and Alzheimer’s disease individuals from cortical sources of resting state EEG rhythms. Front. Neurosci. 2016;10:47. doi: 10.3389/fnins.2016.00047. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

