Early neuroimaging and ultrastructural correlates of injury outcome after neonatal hypoxic-ischaemia
- PMID: 33981995
- PMCID: PMC8103732
- DOI: 10.1093/braincomms/fcab048
Early neuroimaging and ultrastructural correlates of injury outcome after neonatal hypoxic-ischaemia
Erratum in
-
Corrigendum to: Early neuroimaging and ultrastructural correlates of injury outcome after neonatal hypoxic-ischaemia.Brain Commun. 2021 Sep 14;3(3):fcab153. doi: 10.1093/braincomms/fcab153. eCollection 2021. Brain Commun. 2021. PMID: 34671725 Free PMC article.
Abstract
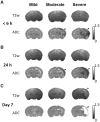
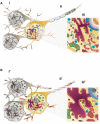
Hypoxic ischaemia encephalopathy is the major cause of brain injury in new-borns. However, to date, useful biomarkers which may be used to early predict neurodevelopmental impairment for proper commencement of hypothermia therapy is still lacking. This study aimed to determine whether the early neuroimaging characteristics and ultrastructural correlates were associated with different injury progressions and brain damage severity outcomes after neonatal hypoxic ischaemia. Longitudinal 7 T MRI was performed within 6 h, 24 h and 7 days after hypoxic ischaemia in rat pups. The brain damage outcome at 7 days post-hypoxic ischaemia assessed using histopathology and MRI were classified as mild, moderate and severe. We found there was a spectrum of different brain damage severity outcomes after the same duration of hypoxic ischaemia. The severity of brain damage determined using MRI correlated well with that assessed by histopathology. Quantitative MRI characteristics denoting water diffusivity in the tissue showed significant differences in the apparent diffusion coefficient deficit volume and deficit ratios within 6 h, at 24 h and 7 days after hypoxic ischaemia among the 3 different outcome groups. The susceptible brain areas to hypoxic ischaemia were revealed by the temporal changes in regional apparent diffusion coefficient values among three outcome groups. Within 6 h post-hypoxic ischaemia, a larger apparent diffusion coefficient deficit volume and deficit ratios and lower apparent diffusion coefficient values were highly associated with adverse brain damage outcome. In the apparent diffusion coefficient deficit areas detected early after hypoxic ischaemia which were highly associated with severe damage outcome, transmission electron microscopy revealed fragmented nuclei; swollen rough endoplasmic reticulum and degenerating mitochondria in the cortex and prominent myelin loss and axon detraction in the white matter. Taken together, different apparent diffusion coefficient patterns obtained early after hypoxic ischaemia are highly associated with different injury progression leading to different brain damage severity outcomes, suggesting the apparent diffusion coefficient characteristics may be applicable to early identify the high-risk neonates for hypothermia therapy.
Keywords: apparent diffusion coefficient; hypoxic-ischaemia; magnetic resonance imaging; rat pups; transmission electric spectroscopy.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
