Impact of manganese amino acid complex on tissue-specific trace mineral distribution and corpus luteum function in gilts
- PMID: 33982089
- PMCID: PMC8407489
- DOI: 10.1093/jas/skab155
Impact of manganese amino acid complex on tissue-specific trace mineral distribution and corpus luteum function in gilts
Abstract
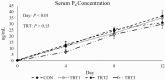
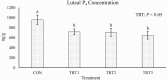
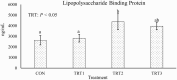
Functional corpora lutea (CL) are required for pregnancy establishment and gestational maintenance in swine, and CL function is susceptible to environmental influences. Manganese (Mn) could be critical in regulating CL function since it is a component of the antioxidant enzyme Mn superoxide dismutase (MnSOD) as well as enzymes involved in cholesterol and steroid hormone synthesis. We hypothesized that a more bioavailable dietary Mn source would increase Mn content in the CL thereby influencing luteal function during the mid-luteal phase of the estrous cycle. Postpubertal gilts (n = 32) were assigned to one of four gestation diets. The control diet (CON) met or exceeded National Research Council (2012) requirements and was formulated to contain 20 parts per million (ppm) of added Mn in the form of Mn sulfate. Three additional diets included 20 (treatment [TRT]1), 40 (TRT2), or 60 (TRT3) ppm of added Mn from a Mn-amino acid complex (Availa-Mn; Zinpro Corporation) instead of Mn sulfate. Dietary treatment began at estrus synchronization onset and continued through 12 days post estrus (dpe) of the ensuing estrous cycle. Blood samples were collected at estrus onset, which was assigned as 0 dpe, as well as 4, 8, and 12 dpe. Gilts were euthanized and tissues were collected at 12 dpe. Serum progesterone (P4) increased (P < 0.01) from 0 to 12 dpe but was unaffected by dietary treatment (P = 0.15) and there was no effect of the interaction between day and treatment (P = 0.85). Luteal Mn content increased (P ≤ 0.05) by 19%, 21%, and 24% in gilts fed TRT1, TRT2, and TRT3, respectively, compared to CON. Luteal P4 concentrations decreased (P = 0.03) 25%, 26%, and 32% in gilts fed TRT1, TRT2, and TRT3, respectively, compared to CON. Relative to CON gilts, CL calcium content decreased (P = 0.02) by 36%, 24%, and 34% for TRT1, TRT2, and TRT3 gilts, respectively. Collectively, these data support the hypothesis that feeding a more bioavailable Mn source increases Mn accumulation in CL tissue. If and how this influences CL function may be related to altered luteal P4 concentrations.
Keywords: corpus luteum; manganese; pig; progesterone; reproduction; trace mineral.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Acda, S. P., and Chae B. J.. . 2002. A review on the applications of organic trace minerals in pig nutrition. Pakistan J. Nutr. 1: 25–30. doi:10.3923/pjn.2002.25.30 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

