Pharmacokinetics of Ruxolitinib in Patients with Atopic Dermatitis Treated With Ruxolitinib Cream: Data from Phase II and III Studies
- PMID: 33982267
- PMCID: PMC8200345
- DOI: 10.1007/s40257-021-00610-x
Pharmacokinetics of Ruxolitinib in Patients with Atopic Dermatitis Treated With Ruxolitinib Cream: Data from Phase II and III Studies
Abstract
Background: Pathogenesis of atopic dermatitis (AD) involves the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway. A cream formulation of ruxolitinib, a potent selective JAK1/JAK2 inhibitor, was developed for topical delivery.
Method: Pharmacokinetic data were obtained from three double-blind, vehicle-controlled studies in patients with AD: a phase II study with ruxolitinib cream 0.15%, 0.5%, or 1.5% once daily or 1.5% twice daily (BID), and two phase III studies with 0.75% or 1.5% BID. Effects of baseline characteristics on pharmacokinetics were examined. Correlations were attempted between plasma concentrations and change in hematological parameters over time.
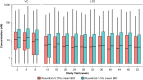
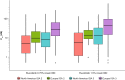
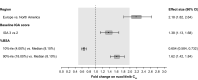
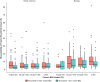
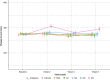
Results: Ruxolitinib plasma concentrations at steady-state (Css) increased with cream strength in a less-than-dose-proportional manner. In the phase III studies, overall mean (standard deviation [SD]) Css after ruxolitinib cream 0.75% and 1.5% BID (23.8 [35.0] and 35.7 [55.0] nM) were a fraction of the half-maximal inhibitory concentration for thrombopoietin-stimulated phosphorylated STAT3 inhibition (281 nM), a JAK/STAT signaling marker. Three covariates were identified for Css: dose, percent body surface area (%BSA) treated, and baseline Investigator's Global Assessment score. Mean (SD) bioavailability of ruxolitinib cream 1.5% BID was 6.22% (7.66%). There were no correlations between Css and any hematological changes except for a transient increase in platelets at week 2.
Conclusions: Plasma ruxolitinib concentrations after treatment with topical ruxolitinib cream in patients with up to 20% BSA affected by AD are not expected to lead to systemic plasma concentrations that may be associated with adverse effects commonly associated with oral JAK inhibitors. CLINICALTRIALS.GOV: NCT03011892; NCT03745638; NCT03745651.
Conflict of interest statement
All authors are employees and shareholders of Incyte Corporation.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

