Multi-institutional, prospective, randomized, double-blind, placebo-controlled phase IIb trial of the tumor lysate, particle-loaded, dendritic cell (TLPLDC) vaccine to prevent recurrence in high-risk melanoma patients: A subgroup analysis
- PMID: 33982452
- PMCID: PMC8267143
- DOI: 10.1002/cam4.3969
Multi-institutional, prospective, randomized, double-blind, placebo-controlled phase IIb trial of the tumor lysate, particle-loaded, dendritic cell (TLPLDC) vaccine to prevent recurrence in high-risk melanoma patients: A subgroup analysis
Abstract
Background: Checkpoint inhibitors (CPI) in combination with cell-based vaccines may produce synergistic antitumor immunity. The primary analysis of the randomized and blinded phase IIb trial in resected stage III/IV melanoma demonstrated TLPLDC is safe and improved 24-month disease-free survival (DFS) in the per treatment (PT) analysis. Here, we examine efficacy within pre-specified and exploratory subgroups.
Methods: Stage III/IV patients rendered disease-free by surgery were randomized 2:1 to TLPLDC vaccine versus placebo. The pre-specified PT analysis included only patients completing the primary vaccine/placebo series at 6 months. Kaplan-Meier analysis was used to compare 24-month DFS among subgroups.
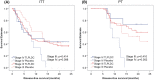
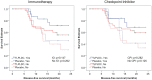
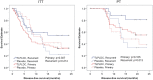
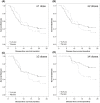
Results: There were no clinicopathologic differences between subgroups except stage IV patients were more likely to receive CPI. In stage IV patients, 24-month DFS was 43% for vaccine versus 0% for placebo (p = 0.098) in the ITT analysis and 73% versus 0% (p = 0.002) in the PT analysis. There was no significant difference in 24-month DFS when stratified by use of immunotherapy or CPI. For patients with resected recurrent disease, 24-month DFS was 88.9% versus 33.3% (p = 0.013) in the PT analysis. All benefit from vaccination was in the PT analysis; no benefit was found in patients receiving up to three doses.
Conclusion: The TLPLDC vaccine improved DFS in patients completing the primary vaccine series, particularly in the resected stage IV patients. The efficacy of the TLPLDC vaccine will be confirmed in a phase III study evaluating adjuvant TLPLDC + CPI versus Placebo + CPI in resected stage IV melanoma patients.
Keywords: cancer vaccine; immunotherapy; melanoma; personalized medicine.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Faries is an advisor for Bristol Myers‐Squibb, Sanofi, Array Bioscience, and Pulse Bioscience. Dr. Hyngstrom has received funding from Castle Biosciences and Amgen. Dr. Berger has received funding from Castle Biosciences and Cardinal Health. Ms Park and Ms Sloan are employed by Orbis Health Solutions. Dr. Wagner is employed by Orbis Health Solutions and Perseus PCI and has received funding from Elios Therapeutics. Dr. Peoples is employed by Orbis Health Solutions and Cancer Insight; is a consultant for Rapamycin Holdings, Heat Biologics, Abexxa Biologics, and Pelican Therapeutics; and has received funding from the above as well as Sellas Life Sciences and Genentech.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

