Interleukin-34 Reprograms Glycolytic and Osteoclastic Rheumatoid Arthritis Macrophages via Syndecan 1 and Macrophage Colony-Stimulating Factor Receptor
- PMID: 33982895
- PMCID: PMC8568622
- DOI: 10.1002/art.41792
Interleukin-34 Reprograms Glycolytic and Osteoclastic Rheumatoid Arthritis Macrophages via Syndecan 1 and Macrophage Colony-Stimulating Factor Receptor
Abstract
Objective: In rheumatoid arthritis (RA), elevated serum interleukin-34 (IL-34) levels are linked with increased disease severity. IL-34 binds to 2 receptors, macrophage colony-stimulating factor receptor (M-CSFR) and syndecan 1, which are coexpressed in RA macrophages. Expression of both IL-34 and syndecan 1 is strikingly elevated in the RA synovium, yet their mechanisms of action remain undefined. This study was undertaken to investigate the mechanism of action of IL-34 in RA.
Methods: To characterize the significance of IL-34 in immunometabolism, its mechanism of action was elucidated in joint macrophages, fibroblasts, and T effector cells using RA and preclinical models.
Results: Intriguingly, syndecan 1 activated IL-34-induced M-CSFR phosphorylation and reprogrammed RA naive cells into distinctive CD14+CD86+GLUT1+ M34 macrophages that expressed elevated levels of IL-1β, CXCL8, and CCL2. In murine M34 macrophages, the inflammatory phenotype was accompanied by potentiated glycolytic activity, exhibited by transcriptional up-regulation of GLUT1, c-Myc, and hypoxia-inducible factor 1α (HIF-1α) and amplified pyruvate and l-lactate secretion. Local expression of IL-34 provoked arthritis by expanding the glycolytic F4/80-positive, inducible nitric oxide synthase (iNOS)-positive macrophage population, which in turn attracted fibroblasts and polarized Th1/Th17 cells. The cross-talk between murine M34 macrophages and Th1/Th17 cells broadened the inflammatory and metabolic phenotypes, resulting in the expansion of IL-34 pathogenicity. Consequently, IL-34-instigated joint inflammation was alleviated in RAG-/- mice compared to wild-type mice. Syndecan 1 deficiency attenuated IL-34-induced arthritis by interfering with joint glycolytic M34 macrophage and osteoclast remodeling. Similarly, inhibition of glycolysis by 2-deoxy-d-glucose reversed the joint swelling and metabolic rewiring triggered by IL-34 via HIF-1α and c-Myc induction.
Conclusion: IL-34 is a novel endogenous factor that remodels hypermetabolic M34 macrophages and facilitates their cross-regulation with T effector cells to advance inflammatory bone destruction in RA.
© 2021, American College of Rheumatology.
Conflict of interest statement
Figures

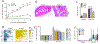

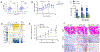
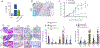

References
-
- McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis - shaping the immunological landscape. Nature Reviews: Rheumatology 2016;12:63–8. - PubMed
-
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. - PubMed
-
- Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum 1996;39:115–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

