High Aspect Ratio and Light-Sensitive Micropillars Based on a Semiconducting Polymer Optically Regulate Neuronal Growth
- PMID: 33983012
- PMCID: PMC8161421
- DOI: 10.1021/acsami.1c03537
High Aspect Ratio and Light-Sensitive Micropillars Based on a Semiconducting Polymer Optically Regulate Neuronal Growth
Abstract
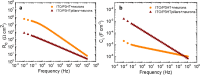
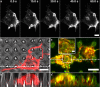
Many nano- and microstructured devices capable of promoting neuronal growth and network formation have been previously investigated. In certain cases, topographical cues have been successfully complemented with external bias, by employing electrically conducting scaffolds. However, the use of optical stimulation with topographical cues was rarely addressed in this context, and the development of light-addressable platforms for modulating and guiding cellular growth and proliferation remains almost completely unexplored. Here, we develop high aspect ratio micropillars based on a prototype semiconducting polymer, regioregular poly(3-hexylthiophene-2,5-diyl) (P3HT), as an optically active, three-dimensional platform for embryonic cortical neurons. P3HT micropillars provide a mechanically compliant environment and allow a close contact with neuronal cells. The combined action of nano/microtopography and visible light excitation leads to effective optical modulation of neuronal growth and orientation. Embryonic neurons cultured on polymer pillars show a clear polarization effect and, upon exposure to optical excitation, a significant increase in both neurite and axon length. The biocompatible, microstructured, and light-sensitive platform developed here opens up the opportunity to optically regulate neuronal growth in a wireless, repeatable, and spatio-temporally controlled manner without genetic modification. This approach may be extended to other cell models, thus uncovering interesting applications of photonic devices in regenerative medicine.
Keywords: cell optical excitation; cell−substrate interface; conjugated polymers; embryonic cortical neurons; microstructured cell interfaces; tissue engineering; topography.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Franze K.; Guck J. The Biophysics of Neuronal Growth. Rep. Prog. Phys. 2010, 73, 094601. 10.1088/0034-4885/73/9/094601. - DOI
-
- Fu L.; Xie J.; Carlson M. A.; Reilly D. A. Three-Dimensional Nanofiber Scaffolds with Arrayed Holes for Engineering Skin Tissue Constructs. MRS Commun. 2017, 7, 361–366. 10.1557/mrc.2017.49. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

