Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial
- PMID: 33983065
- PMCID: PMC8127799
- DOI: 10.1177/03000605211013550
Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial
Abstract
Objective: We evaluated whether ivermectin combined with doxycycline reduced the clinical recovery time in adults with COVID-19 infection.
Methods: This was a randomized, blinded, placebo-controlled trial in patients with mild-to-moderate COVID-19 symptoms randomly assigned to treatment (n = 200) and placebo (n = 200) groups. The primary outcome was duration from treatment to clinical recovery. Secondary outcomes were disease progression and persistent COVID-19 positivity by RT-PCR.
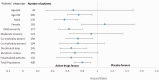
Results: Among 556 screened patients, 400 were enrolled and 363 completed follow-up. The mean patient age was 40 years, and 59% were men. The median recovery time was 7 (4-10, treatment group) and 9 (5-12, placebo group) days (hazard ratio, 0.73; 95% confidence interval, 0.60-0.90). The number of patients with a ≤7-day recovery was 61% (treatment group) and 44% (placebo groups) (hazard ratio, 0.06; 95% confidence interval, 0.04-0.09). The proportion of patients who remained RT-PCR positive on day 14 and whose disease did not progress was significantly lower in the treatment group than in the placebo group.
Conclusions: Patients with mild-to-moderate COVID-19 infection treated with ivermectin plus doxycycline recovered earlier, were less likely to progress to more serious disease, and were more likely to be COVID-19 negative by RT-PCR on day 14.
Trial registration: ClinicalTrials.gov Identifier: NCT04523831.
Data repository id: Dryad. doi:10.5061/dryad.qjq2bvqf6.
Keywords: COVID-19; Ivermectin; doxycycline; infection; recovery time; reverse transcription polymerase chain reaction.
Conflict of interest statement
Figures

References
-
- World Health Organization. GCM teleconference – Note for the Records. 10 January 2020. Subject: Pneumonia in Wuhan, China. Accessed November 1, 2020. https://www. who. int/blueprint/10-01-2020-nfr-gcm.pdf?ua = .
-
- WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Accessed November 1, 2020.: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re....
-
- World Health Organization. International Clinical Trial Registry Platform. COVID19 trials. Accessed November 1, 2020. https://www.who.int/ictrp/en/.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

