Propensity for somatic expansion increases over the course of life in Huntington disease
- PMID: 33983118
- PMCID: PMC8118653
- DOI: 10.7554/eLife.64674
Propensity for somatic expansion increases over the course of life in Huntington disease
Abstract
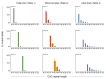
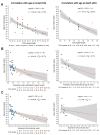
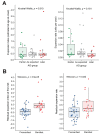
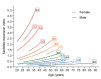
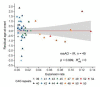
Recent work on Huntington disease (HD) suggests that somatic instability of CAG repeat tracts, which can expand into the hundreds in neurons, explains clinical outcomes better than the length of the inherited allele. Here, we measured somatic expansion in blood samples collected from the same 50 HD mutation carriers over a twenty-year period, along with post-mortem tissue from 15 adults and 7 fetal mutation carriers, to examine somatic expansions at different stages of life. Post-mortem brains, as previously reported, had the greatest expansions, but fetal cortex had virtually none. Somatic instability in blood increased with age, despite blood cells being short-lived compared to neurons, and was driven mostly by CAG repeat length, then by age at sampling and by interaction between these two variables. Expansion rates were higher in symptomatic subjects. These data lend support to a previously proposed computational model of somatic instability-driven disease.
Keywords: CAG expansion; HD mutation carrier; Huntington disease; genetics; genomics; human; human data; longitudinal study; neuroscience; somatic instability.
© 2021, Kacher et al.
Conflict of interest statement
RK, FL, SN, CC, AB, SH No competing interests declared, AD serves on the advisory boards of Triplet Therapeutics. and holds partly a Patent B 06291873.5, "Anaplerotic therapy of HD and other polyglutamine diseases."
Figures



References
-
- Andrew SE, Paul Goldberg Y, Kremer B, Telenius H, Theilmann J, Adam S, Starr E, Squitieri F, Lin B, Kalchman MA, Graham RK, Hayden MR. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nature Genetics. 1993;4:398–403. doi: 10.1038/ng0893-398. - DOI - PubMed
-
- Angeles-López QD, García-Lara L, Aguirre-Pineda N, Castañeda-Arellano R, Elizondo-Azuela G, Pérez-Severiano F, Segovia J. The absence of the aryl hydrocarbon receptor in the R6/1 transgenic mouse model of Huntington's disease improves the neurological phenotype. Behavioural Brain Research. 2021;408:113230. doi: 10.1016/j.bbr.2021.113230. - DOI - PubMed
-
- Barnat M, Capizzi M, Aparicio E, Boluda S, Wennagel D, Kacher R, Kassem R, Lenoir S, Agasse F, Braz BY, Liu J-P, Ighil J, Tessier A, Zeitlin SO, Duyckaerts C, Dommergues M, Durr A, Humbert S. Huntington’s disease alters human neurodevelopment. Science. 2020;369:787–793. doi: 10.1126/science.aax3338. - DOI - PMC - PubMed
-
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear Mixed-Effects models using lme4. Journal of Statistical Software. 2015a;67:1–48. doi: 10.18637/jss.v067.i01. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

