TRIM37 prevents formation of condensate-organized ectopic spindle poles to ensure mitotic fidelity
- PMID: 33983387
- PMCID: PMC8127006
- DOI: 10.1083/jcb.202010180
TRIM37 prevents formation of condensate-organized ectopic spindle poles to ensure mitotic fidelity
Abstract
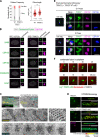
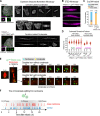
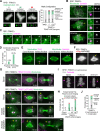
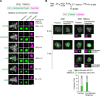
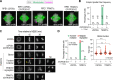
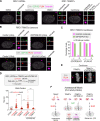
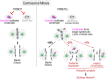
Centrosomes are composed of a centriolar core surrounded by pericentriolar material that nucleates microtubules. The ubiquitin ligase TRIM37 localizes to centrosomes, but its centrosomal roles are not yet defined. We show that TRIM37 does not control centriole duplication, structure, or the ability of centrioles to form cilia but instead prevents assembly of an ectopic centrobin-scaffolded structured condensate that forms by budding off of centrosomes. In ∼25% of TRIM37-deficient cells, the condensate organizes an ectopic spindle pole, recruiting other centrosomal proteins and acquiring microtubule nucleation capacity during mitotic entry. Ectopic spindle pole-associated transient multipolarity and multipolar segregation in TRIM37-deficient cells are suppressed by removing centrobin, which interacts with and is ubiquitinated by TRIM37. Thus, TRIM37 ensures accurate chromosome segregation by preventing the formation of centrobin-scaffolded condensates that organize ectopic spindle poles. Mutations in TRIM37 cause the disorder mulibrey nanism, and patient-derived cells harbor centrobin condensate-organized ectopic poles, leading us to propose that chromosome missegregation is a pathological mechanism in this disorder.
© 2021 Meitinger et al.
Figures








References
-
- Aziz, K., Sieben C.J., Jeganathan K.B., Hamada M., Davies B.A., Velasco R.O.F., Rahman N., Katzmann D.J., and van Deursen J.M.. 2018. Mosaic-variegated aneuploidy syndrome mutation or haploinsufficiency in Cep57 impairs tumor suppression. J. Clin. Invest. 128:3517–3534. 10.1172/JCI120316 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

