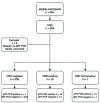
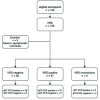
Evaluation of the test accuracy of a SARS-CoV-2 rapid antigen test in symptomatic community dwelling individuals in the Netherlands
- PMID: 33983971
- PMCID: PMC8118553
- DOI: 10.1371/journal.pone.0250886
Evaluation of the test accuracy of a SARS-CoV-2 rapid antigen test in symptomatic community dwelling individuals in the Netherlands
Abstract
Background: SARS-CoV-2 real-time reverse transcriptase polymerase chain reaction (qRT-PCR) is well suited for the diagnosis of clinically ill patients requiring treatment. Application for community testing of symptomatic individuals for disease control purposes however raises challenges. SARS-CoV-2 rapid antigen tests might offer an alternative, but quality evidence on their performance is limited.
Methods: We conducted an evaluation of the test accuracy of the 'BD Veritor System for Rapid Detection of SARS-CoV-2' (VRD) compared to qRT-PCR on combined nose/throat swabs obtained from symptomatic individuals at Municipal Health Service (MHS) COVID-19 test centers in the Netherlands. In part one of the study, with the primary objective to evaluate test sensitivity and specificity, all adults presenting at one MHS test center were eligible for inclusion. In part two, with the objective to evaluate test sensitivity stratified by Ct (cycle threshold)-value and time since symptom onset, adults who had a positive qRT-PCR obtained at a MHS test center were eligible.
Findings: In part one (n = 352) SARS-CoV-2 prevalence was 4.8%, overall specificity 100% (95%CI: 98·9%-100%) and sensitivity 94·1% (95%CI: 71·1%-100%). In part two (n = 123) the sensitivity was 78·9% (95%CI: 70·6%-85·7%) overall, 89·4% (95% CI: 79·4%-95·6%) for specimen obtained within seven days after symptom onset and 93% (95% CI: 86%-97.1%) for specimen with a Ct-value below 30.
Interpretation: The VRD is a promising diagnostic for COVID-19 testing of symptomatic community-dwelling individuals within seven days after symptom onset in context of disease control. Further research on practical applicability and the optimal position within the testing landscape is needed.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- European Centre for Disease Prevention and Control. COVID-19 testing strategies and objectives. 2020 Sep 15. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/TestingStrategy...
-
- Becton, Dickinson and Company. BD Veritor System for Rapid Detection of SARS-CoV-2 Package Insert. 2020
-
- Roche Holding. Roche SARS-CoV-2 Rapid Antigen test Package Insert. 2020
-
- Dinnes J, Deeks JJ, Adriano A, Berhane S, Davenport C, Dittrich S et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020. August 26;8 10.1002/14651858.CD013705 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous