Compartmentalization of cerebrospinal fluid inflammation across the spectrum of untreated HIV-1 infection, central nervous system injury and viral suppression
- PMID: 33983973
- PMCID: PMC8118251
- DOI: 10.1371/journal.pone.0250987
Compartmentalization of cerebrospinal fluid inflammation across the spectrum of untreated HIV-1 infection, central nervous system injury and viral suppression
Abstract
Objective: To characterize the evolution of central nervous system (CNS) inflammation in HIV-1 infection applying a panel of cerebrospinal fluid (CSF) inflammatory biomarkers to grouped subjects representing a broad spectrum of systemic HIV-1 immune suppression, CNS injury and viral control.
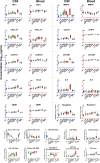
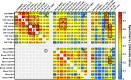
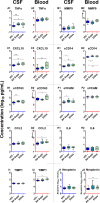
Methods: This is a cross-sectional analysis of archived CSF and blood samples, assessing concentrations of 10 functionally diverse soluble inflammatory biomarkers by immunoassays in 143 HIV-1-infected subjects divided into 8 groups: untreated primary HIV-1 infection (PHI); four untreated groups defined by their blood CD4+ T lymphocyte counts; untreated patients presenting with subacute HIV-associated dementia (HAD); antiretroviral-treated subjects with ≥1 years of plasma viral suppression; and untreated elite controllers. Twenty HIV-1-uninfected controls were included for comparison. Background biomarkers included blood CD4+ and CD8+ T lymphocytes, CSF and blood HIV-1 RNA, CSF white blood cell (WBC) count, CSF/blood albumin ratio, CSF neurofilament light chain (NfL), and CSF t-tau.
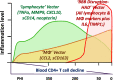
Findings: HIV-1 infection was associated with a broad compartmentalized CSF inflammatory response that developed early in its course and changed with systemic disease progression, development of neurological injury, and viral suppression. CSF inflammation in untreated individuals without overt HAD exhibited at least two overall patterns of inflammation as blood CD4+ T lymphocytes decreased: one that peaked at 200-350 blood CD4+ T cells/μL and associated with lymphocytic CSF inflammation and HIV-1 RNA concentrations; and a second that steadily increased through the full range of CD4+ T cell decline and associated with macrophage responses and increasing CNS injury. Subacute HAD was distinguished by a third inflammatory profile with increased blood-brain barrier permeability and robust combined lymphocytic and macrophage CSF inflammation. Suppression of CSF and blood HIV-1 infections by antiretroviral treatment and elite viral control were associated with reduced CSF inflammation, though not fully to levels found in HIV-1 seronegative controls.
Conflict of interest statement
The authors have no competing interests that influenced the contents of this paper. However, the authors list the following general potential conflicts of interest: RWP had been a consultant to Merck and Co and had received an honorarium and travel support from AbbVie and Gilead Sciences for meeting presentations during part of the time of sample collections. MG has received research grants from Abbott, Baxter, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck, Pfizer, Roche and Tibotec, and he has received honoraria as a speaker and/or scientific advisor from Abbott/Abbvie, Amgen, Biogen, Bioinvent, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen-Cilag, MSD, Novocure, Novo Nordic, Pfizer, Roche and Tibotec. HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. Dr. Spudich has received an honorarium and travel support from AbbVie, Inc. for a meeting presentation. BLS has received research grants from Gilead Sciences, and an honorarium and travel support from Merck. SMK, SSS, VA, SS, CDG, DF, LH, JP, PJN, BLS and CTY report no conflicts. In no case were the above-listed activities related directly to the submitted work—neither to the conceptualization, study design, data collection, analysis, or manuscript preparation. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

