Evaluation of the effects of differences in silicone hardness on rat model of lumbar spinal stenosis
- PMID: 33984013
- PMCID: PMC8118556
- DOI: 10.1371/journal.pone.0251464
Evaluation of the effects of differences in silicone hardness on rat model of lumbar spinal stenosis
Abstract
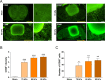
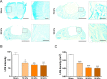
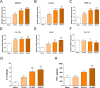
Lumbar spinal stenosis (LSS), one of the most commonly reported spinal disorders, can cause loss of sensation and dyskinesia. In currently used animal models of LSS, the spinal cord is covered entirely with a silicone sheet, or block-shaped silicone is inserted directly into the spinal canal after laminectomy. However, the effects of differences between these implant materials have not been studied. We assessed the degree of damage and locomotor function of an LSS model in Sprague-Dawley rats using silicone blocks of varying hardness (70, 80, and 90 kPa) implanted at the L4 level. In sham rats, the spinal cord remained intact; in LSS rats, the spinal cord was increasingly compressed by the mechanical pressure of the silicone blocks as hardness increased. Inflammatory cells were not evident in sham rats, but numerous inflammatory cells were observed around the implanted silicone block in LSS rats. CD68+ cell quantification revealed increases in the inflammatory response in a hardness-dependent manner in LSS rats. Compared with those in sham rats, proinflammatory cytokine levels were significantly elevated in a hardness-dependent manner, and locomotor function was significantly decreased, in LSS rats. Overall, this study showed that hardness could be used as an index to control the severity of nerve injury induced by silicone implants.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Simvastatin ameliorates cauda equina compression injury in a rat model of lumbar spinal stenosis.J Neuroimmune Pharmacol. 2013 Mar;8(1):274-86. doi: 10.1007/s11481-012-9419-3. Epub 2012 Nov 28. J Neuroimmune Pharmacol. 2013. PMID: 23188522 Free PMC article.
-
Clinical characteristics and surgical outcome of the symptomatic ossification of ligamentum flavum at the thoracic level with combined lumbar spinal stenosis.Arch Orthop Trauma Surg. 2012 Apr;132(4):465-70. doi: 10.1007/s00402-011-1438-7. Epub 2011 Dec 3. Arch Orthop Trauma Surg. 2012. PMID: 22139388
-
Relationship between lumbar spinal stenosis and axial muscle wasting.Spine J. 2024 Feb;24(2):231-238. doi: 10.1016/j.spinee.2023.09.020. Epub 2023 Oct 1. Spine J. 2024. PMID: 37788745
-
The clinical significance of redundant nerve roots of the cauda equina in lumbar spinal stenosis patients: A systematic literature review and meta-analysis.Clin Neurol Neurosurg. 2018 Nov;174:40-47. doi: 10.1016/j.clineuro.2018.09.001. Epub 2018 Sep 4. Clin Neurol Neurosurg. 2018. PMID: 30205275
-
Application of X STOP device in the treatment of lumbar spinal stenosis.Pain Physician. 2010 Sep-Oct;13(5):E327-36. Pain Physician. 2010. PMID: 20859324 Review.
Cited by
-
Human Placental Extract as a Promising Epidural Therapy for Lumbar Spinal Stenosis: Enhancing Axonal Plasticity and Mitigating Pain and Inflammation in a Rat Model.JOR Spine. 2025 Jun 17;8(2):e70085. doi: 10.1002/jsp2.70085. eCollection 2025 Jun. JOR Spine. 2025. PMID: 40535717 Free PMC article.
-
From the Matrix to the Nucleus and Back: Mechanobiology in the Light of Health, Pathologies, and Regeneration of Oral Periodontal Tissues.Biomolecules. 2021 May 31;11(6):824. doi: 10.3390/biom11060824. Biomolecules. 2021. PMID: 34073044 Free PMC article. Review.
-
Animal Models of Intervertebral Disc Diseases: Advantages, Limitations, and Future Directions.Neurol Int. 2024 Dec 9;16(6):1788-1818. doi: 10.3390/neurolint16060129. Neurol Int. 2024. PMID: 39728755 Free PMC article. Review.
-
Compensatory upregulation of MT2A alleviates neurogenic intermittent claudication through inhibiting activated p38 MAPK-mediated neuronal apoptosis.Hum Cell. 2024 May;37(3):675-688. doi: 10.1007/s13577-024-01043-4. Epub 2024 Mar 28. Hum Cell. 2024. PMID: 38546949
-
Transforming Growth Factor-β Induces Interleukin-6 Secretion from Human Ligamentum Flavum-Derived Cells through Partial Activation of p38 and p44/42 Mitogen-Activated Protein Kinases.Asian Spine J. 2023 Dec;17(6):997-1003. doi: 10.31616/asj.2023.0025. Epub 2023 Nov 10. Asian Spine J. 2023. PMID: 37946333 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

