The peptidoglycan-associated protein NapA plays an important role in the envelope integrity and in the pathogenesis of the lyme disease spirochete
- PMID: 33984073
- PMCID: PMC8118282
- DOI: 10.1371/journal.ppat.1009546
The peptidoglycan-associated protein NapA plays an important role in the envelope integrity and in the pathogenesis of the lyme disease spirochete
Abstract
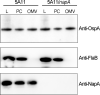
The bacterial pathogen responsible for causing Lyme disease, Borrelia burgdorferi, is an atypical Gram-negative spirochete that is transmitted to humans via the bite of an infected Ixodes tick. In diderms, peptidoglycan (PG) is sandwiched between the inner and outer membrane of the cell envelope. In many other Gram-negative bacteria, PG is bound by protein(s), which provide both structural integrity and continuity between envelope layers. Here, we present evidence of a peptidoglycan-associated protein (PAP) in B. burgdorferi. Using an unbiased proteomics approach, we identified Neutrophil Attracting Protein A (NapA) as a PAP. Interestingly, NapA is a Dps homologue, which typically functions to bind and protect cellular DNA from damage during times of stress. While B. burgdorferi NapA is known to be involved in the oxidative stress response, it lacks the critical residues necessary for DNA binding. Biochemical and cellular studies demonstrate that NapA is localized to the B. burgdorferi periplasm and is indeed a PAP. Cryo-electron microscopy indicates that mutant bacteria, unable to produce NapA, have structural abnormalities. Defects in cell-wall integrity impact growth rate and cause the napA mutant to be more susceptible to osmotic and PG-specific stresses. NapA-linked PG is secreted in outer membrane vesicles and augments IL-17 production, relative to PG alone. Using microfluidics, we demonstrate that NapA acts as a molecular beacon-exacerbating the pathogenic properties of B. burgdorferi PG. These studies further our understanding of the B. burgdorferi cell envelope, provide critical information that underlies its pathogenesis, and highlight how a highly conserved bacterial protein can evolve mechanistically, while maintaining biological function.
Conflict of interest statement
All authors declare no competing interests.
Figures




References
-
- Prevention CfDCa. Recent Lyme disease surveillance data November 7, 2019. [Available from: https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

