Confined placental mosaicism and the association with pregnancy outcome and fetal growth: a review of the literature
- PMID: 33984128
- PMCID: PMC8382909
- DOI: 10.1093/humupd/dmab009
Confined placental mosaicism and the association with pregnancy outcome and fetal growth: a review of the literature
Abstract
Background: Chromosomal mosaicism can be detected in different stages of early life: in cleavage stage embryos, in blastocysts and biopsied cells from blastocysts during preimplantation genetic testing for aneuploidies (PGT-A) and later during prenatal testing, as well as after birth in cord blood. Mosaicism at all different stages can be associated with adverse pregnancy outcomes. There is an onward discussion about whether blastocysts diagnosed as chromosomally mosaic by PGT-A should be considered safe for transfer. An accurate diagnosis of mosaicism remains technically challenging and the fate of abnormal cells within an embryo remains largely unknown. However, if aneuploid cells persist in the extraembryonic tissues, they can give rise to confined placental mosaicism (CPM). Non-invasive prenatal testing (NIPT) uses cell-free (cf) DNA released from the placenta in maternal blood, facilitating the detection of CPM. In literature, conflicting evidence is found about whether CPM is associated with fetal growth restriction (FGR) and/or other pregnancy outcomes. This makes counselling for patients by clinicians challenging and more knowledge is needed for clinical decision and policy making.
Objective and rationale: The objective of this review is to evaluate the association between CPM and prenatal growth and adverse pregnancy outcomes. All relevant literature has been reviewed in order to achieve an overview on merged results exploring the relation between CPM and FGR and other adverse pregnancy outcomes.
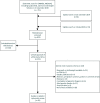
Search methods: The following Medical Subject Headings (MESH) terms and all their synonyms were used: placental, trophoblast, cytotrophoblast, mosaicism, trisomy, fetal growth, birth weight, small for gestational age and fetal development. A search in Embase, PubMed, Medline Ovid, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL) and Google Scholar databases was conducted. Relevant articles published until 16 July 2020 were critically analyzed and discussed.
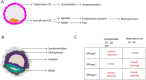
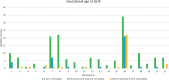
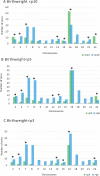
Outcomes: There were 823 articles found and screened based on their title/abstract. From these, 213 articles were selected and full text versions were obtained for a second selection, after which 70 publications were included and 328 cases (fetuses) were analyzed. For CPM in eight different chromosomes (of the total 14 analyzed), there was sufficient evidence that birth weight was often below the 5th percentile of fetal growth standards. FGR was reported in 71.7% of CPM cases and preterm birth (<37 weeks of delivery) was reported in 31.0% of cases. A high rate of structural fetal anomalies, 24.2%, in cases with CPM was also identified. High levels of mosaicism in CVS and presence of uniparental disomy (UPD) were significantly associated with adverse pregnancy outcomes.
Wider implications: Based on the literature, the advice to clinicians is to monitor fetal growth intensively from first trimester onwards in case of CPM, especially when chromosome 2, 3, 7, 13, 15, 16 and 22 are involved. In addition to this, it is advised to examine the fetuses thoroughly for structural fetal anomalies and raise awareness of a higher chance of (possibly extreme) premature birth. Despite prematurity in nearly a fifth of cases, the long-term follow-up of CPM life borns seems to be positive. More understanding of the biological mechanisms behind CPM will help in prioritizing embryos for transfer after the detection of mosaicism in embryos through PGT-A.
Keywords: aneuploidy; birth weight; chorionic villi sampling; embryonic development; fertilization in vitro; fetal growth retardation; mosaicism; pregnancy; pregnancy outcome; trisomy.
© The Author(s) 2021. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures

Comment in
-
The missing role of diagnosis of confined placental mosaicism in the management of fetal growth restriction.Hum Reprod Update. 2021 Dec 21;28(1):149-150. doi: 10.1093/humupd/dmab032. Hum Reprod Update. 2021. PMID: 34642745 No abstract available.
-
Reply: The missing role of diagnosis of confined placental mosaicism in the management of fetal growth restriction.Hum Reprod Update. 2021 Dec 21;28(1):151-152. doi: 10.1093/humupd/dmab033. Hum Reprod Update. 2021. PMID: 34642759 Free PMC article. No abstract available.
References
-
- Akera T, Lampson MA.. Chromosome segregation: poor supervision in the early stage of life. Curr Biol 2019;29:R156–R158. - PubMed
-
- Amor DJ, Neo WT, Waters E, Heussler H, Pertile M, Halliday J.. Health and developmental outcome of children following prenatal diagnosis of confined placental mosaicism. Prenat Diagn 2006;26:443–448. - PubMed
-
- AppelmanZ, , RosensaftJ, , ChemkeJ, , CaspiB, , AshkenaziM, , Mogilner MB.. Trisomy 9 confined to the placenta: prenatal diagnosis and neonatal follow-up. Am J Med Genet 1991;40:464–466. - PubMed
-
- Ariel I, Lerer I, Yagel S, Cohen R, Ben-Neriah Z, Abeliovich D.. Trisomy 2: confined placental mosaicism in a fetus with intrauterine growth retardation. Prenat Diagn 1997;17:180–183. - PubMed
-
- AstnerA, , SchwingerE, , CaliebeA, , JonatW, , Gembruch U.. Sonographically detected fetal and placental abnormalities associated with trisomy 16 confined to the placenta. A case report and review of the literature. Prenatal Diagnosis 1998;18:1308–1315. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

