Regulation of macrophage functions by FABP-mediated inflammatory and metabolic pathways
- PMID: 33984518
- PMCID: PMC8169605
- DOI: 10.1016/j.bbalip.2021.158964
Regulation of macrophage functions by FABP-mediated inflammatory and metabolic pathways
Abstract
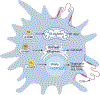
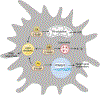
Macrophages are almost everywhere in the body, where they serve pivotal functions in maintaining tissue homeostasis, remodeling, and immunoregulation. Macrophages are traditionally thought to differentiate from bone marrow-derived hematopoietic stem cells (HSCs). Emerging studies suggest that some tissue macrophages at steady state originate from embryonic precursors in the yolk sac or fetal liver and are maintained in situ by self-renewal, but bone marrow-derived monocytes can give rise to tissue macrophages in pathogenic settings, such as inflammatory injuries and cancer. Macrophages are popularly classified as Th1 cytokine (e.g. IFNγ)-activated M1 macrophages (the classical activation) or Th2 cytokine (e.g. IL-4)-activated M2 macrophages (the alternative activation). However, given the myriad arrays of stimuli macrophages may encounter from local environment, macrophages exhibit notorious heterogeneity in their phenotypes and functions. Determining the underlying metabolic pathways engaged during macrophage activation is critical for understanding macrophage phenotypic and functional adaptivity under different disease settings. Fatty acid binding proteins (FABPs) represent a family of evolutionarily conserved proteins facilitating lipid transport, metabolism and responses inside cells. More specifically, adipose-FABP (A-FABP) and epidermal-FABP (E-FABP) are highly expressed in macrophages and play a central role in integrating metabolic and inflammatory pathways. In this review we highlight how A-FABP and E-FABP are respectively upregulated in different subsets of activated macrophages and provide a unique perspective in defining macrophage phenotypic and functional heterogeneity through FABP-regulated lipid metabolic and inflammatory pathways.
Keywords: Fatty acid binding proteins; Inflammation; Macrophages; Obesity.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Interest Statement
There is no conflict of interests for all listed authors.
Figures
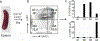

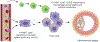
Similar articles
-
Adipocyte Fatty Acid Binding Protein Potentiates Toxic Lipids-Induced Endoplasmic Reticulum Stress in Macrophages via Inhibition of Janus Kinase 2-dependent Autophagy.Sci Rep. 2017 Jan 17;7:40657. doi: 10.1038/srep40657. Sci Rep. 2017. PMID: 28094778 Free PMC article.
-
Pro-inflammatory cytokines negatively regulate PPARγ mediated gene expression in both human and murine macrophages via multiple mechanisms.Immunobiology. 2013 Nov;218(11):1336-44. doi: 10.1016/j.imbio.2013.06.011. Epub 2013 Jul 1. Immunobiology. 2013. PMID: 23870825
-
MicroRNAs: At the Interface of Metabolic Pathways and Inflammatory Responses by Macrophages.Front Immunol. 2020 Aug 14;11:1797. doi: 10.3389/fimmu.2020.01797. eCollection 2020. Front Immunol. 2020. PMID: 32922393 Free PMC article. Review.
-
Fatty acid-binding protein 5 limits the anti-inflammatory response in murine macrophages.Mol Immunol. 2015 Oct;67(2 Pt B):265-75. doi: 10.1016/j.molimm.2015.06.001. Epub 2015 Jun 20. Mol Immunol. 2015. PMID: 26105806 Free PMC article.
-
Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites.Front Immunol. 2021 Nov 5;12:746151. doi: 10.3389/fimmu.2021.746151. eCollection 2021. Front Immunol. 2021. PMID: 34804028 Free PMC article. Review.
Cited by
-
Cutting-Edge CAR Engineering: Beyond T Cells.Biomedicines. 2022 Nov 24;10(12):3035. doi: 10.3390/biomedicines10123035. Biomedicines. 2022. PMID: 36551788 Free PMC article. Review.
-
Structural Properties of Rat Intestinal Fatty Acid-Binding Protein with its Dynamics: Insights into Intrinsic Disorder.Protein Pept Lett. 2024;31(6):458-468. doi: 10.2174/0109298665313811240530055004. Protein Pept Lett. 2024. PMID: 38910419
-
FABP7 in Hepatic Macrophages Promotes Fibroblast Activation and CD4+ T-Cell Migration by Regulating M2 Polarization During Liver Fibrosis.J Immunol Res. 2025 Feb 19;2025:6987981. doi: 10.1155/jimr/6987981. eCollection 2025. J Immunol Res. 2025. PMID: 40017805 Free PMC article.
-
Mechanisms of hepatic and renal injury in lipid metabolism disorders in metabolic syndrome.Int J Biol Sci. 2024 Sep 9;20(12):4783-4798. doi: 10.7150/ijbs.100394. eCollection 2024. Int J Biol Sci. 2024. PMID: 39309427 Free PMC article. Review.
-
Enhanced interpretation of immune cell phenotype and function through a rhesus macaque single-cell atlas.Cell Genom. 2025 May 14;5(5):100849. doi: 10.1016/j.xgen.2025.100849. Epub 2025 Apr 14. Cell Genom. 2025. PMID: 40233746 Free PMC article.
References
-
- Storch J, Corsico B, The emerging functions and mechanisms of mammalian fatty acid-binding proteins, Annu Rev Nutr, 28 (2008) 73–95. - PubMed
-
- Hertzel AV, Bernlohr DA, The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function, Trends Endocrinol Metab, 11 (2000) 175–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

