Deletion of Ulk1 inhibits neointima formation by enhancing KAT2A/GCN5-mediated acetylation of TUBA/α-tubulin in vivo
- PMID: 33985412
- PMCID: PMC8726707
- DOI: 10.1080/15548627.2021.1911018
Deletion of Ulk1 inhibits neointima formation by enhancing KAT2A/GCN5-mediated acetylation of TUBA/α-tubulin in vivo
Abstract
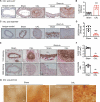
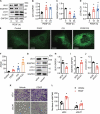
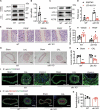
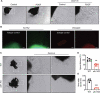
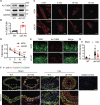
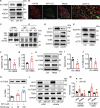
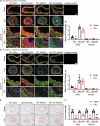
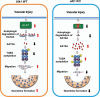
ULK1 (unc-51 like autophagy activating kinase) has a central role in initiating macroautophagy/autophagy, a process that contributes to atherosclerosis and neointima hyperplasia, or excessive tissue growth that leads to vessel dysfunction. However, the role of ULK1 in neointima formation remains unclear. We aimed to determine how Ulk1 deletion affected neointima formation and to investigate the underlying mechanisms. We measured autophagy activity, vascular smooth muscle cell (VSMC) migration and neointima hyperplasia in cultured VSMCs and ligation-injured mouse carotid arteries from male wild-type (WT, C57BL/6 J) and VSMC-specific ulk1 knockout (ulk1 KO) mice. Carotid artery ligation in WT mice increased ULK1 protein expression, and concurrently increased autophagic flux and neointima formation. Treating human aortic smooth muscle cells (HASMCs) with PDGF (platelet derived growth factor) increased ULK1 expression, activated autophagy, and promoted cell migration. Further, smooth muscle cell-specific deletion of Ulk1 suppressed autophagy, inhibited VSMC migration, and impeded neointima hyperplasia. Mechanistically, Ulk1 deletion inhibited autophagic degradation of histone acetyltransferase protein KAT2A/GCN5 (K[lysine] acetyltransferase 2A), resulting in accumulation of KAT2A that directly acetylated TUBA/α-tubulin and subsequently increased protein levels of acetylated TUBA. The acetylation of TUBA increased microtubule stability and inhibited VSMC directional migration and neointima formation. Finally, local transfection of Kat2a siRNA decreased TUBA acetylation and prevented the attenuation of vascular injury-induced neointima formation in ulk1 KO mice. These findings suggest that Ulk1 deletion inhibits neointima formation by reducing autophagic degradation of KAT2A and increasing TUBA acetylation in VSMCs.Abbreviations: ACTA2/α-SMA: actin, alpha 2, smooth muscle, aorta; ACTB: actin beta; ATAT1: alpha tubulin acetyltransferase 1; ATG: autophagy related; BECN1: beclin 1; BP: blood pressure; CAL: carotid artery ligation; CQ: chloroquine diphosphate; EC: endothelial cells; EEL: external elastic layer; FBS: fetal bovine serum; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; HASMCs: human aortic smooth muscle cells; HAT1: histone acetyltransferase 1; HDAC: histone deacetylase; IEL: inner elastic layer; IP: immunoprecipitation; KAT2A/GCN5: K(lysine) acetyltransferase 2A; KAT8/hMOF: lysine acetyltransferase 8; MAP1LC3: microtubule associated protein 1 light chain 3; MYH11: myosin heavy chain 11; PBS: phosphate-buffered saline; PDGF: platelet derived growth factor; PECAM1/CD31: platelet and endothelial cell adhesion molecule 1; RAC3: Rac family small GTPase 3; SIRT2: sirtuin 2; SPP1/OPN: secreted phosphoprotein 1; SQSTM1/p62: sequestosome 1; TAGLN/SM22: transgelin; TUBA: tubulin alpha; ULK1: unc-51 like autophagy activating kinase; VSMC: vascular smooth muscle cell; VVG: Verhoeff Van Gieson; WT: wild type.
Keywords: Autophagy; KAT2A/GCN5; TUBA/α-tubulin acetylation; ULK1; VSMCs; neointima formation.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
References
-
- Mizushima N, Yoshimori T, Ohsumi Y.. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. - PubMed
-
- Martinet W, De Meyer GR. Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res. 2009. Feb;104(3):304–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
