On the Relationship between Spatial Coherence and In Situ Pressure for Abdominal Imaging
- PMID: 33985826
- PMCID: PMC8494065
- DOI: 10.1016/j.ultrasmedbio.2021.03.008
On the Relationship between Spatial Coherence and In Situ Pressure for Abdominal Imaging
Abstract
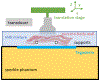
Tissue harmonic signal quality has been shown to improve with elevated acoustic pressure. The peak rarefaction pressure (PRP) for a given transmit, however, is limited by the Food and Drug Administration guidelines for mechanical index. We have previously demonstrated that the mechanical index overestimates in situ PRP for tightly focused beams in vivo, due primarily to phase aberration. In this study, we evaluate two spatial coherence-based image quality metrics-short-lag spatial coherence and harmonic short-lag spatial coherence-as proxy estimates for phase aberration and assess their correlation with in situ PRP in simulations and experiments when imaging through abdominal body walls. We demonstrate strong correlation between both spatial coherence-based metrics and in situ PRP (R2 = 0.77 for harmonic short-lag spatial coherence, R2 = 0.67 for short-lag spatial coherence), an observation that could be leveraged in the future for patient-specific selection of acoustic output.
Keywords: Abdominal ultrasound; In situ pressure; Measurement techniques; Mechanical index; Simulation; Spatial coherence; Tissue harmonic imaging.
Copyright © 2021 World Federation for Ultrasound in Medicine & Biology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest disclosure The authors declare no competing interests.
Figures








References
-
- Asham EH, Kaseb A, Ghobrial RM. Management of Hepatocellular Carcinoma. Surgical Clinics of North America, 2013;93:1423–1450. - PubMed
-
- Bamber JC, Hill CR. Ultrasonic attenuation and propagation speed in mammalian tissues as a function of temperature. Ultrasound in Medicine and Biology, 1979;5:149–157. - PubMed
-
- Bradley C Mechanisms of Image Quality Improvement in Tissue Harmonic Imaging. In: AIP Conference Proceedings. Vol. 838. AIP, 2006. pp. 247–254.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

