Combining AFM13, a Bispecific CD30/CD16 Antibody, with Cytokine-Activated Blood and Cord Blood-Derived NK Cells Facilitates CAR-like Responses Against CD30+ Malignancies
- PMID: 33986022
- PMCID: PMC8254785
- DOI: 10.1158/1078-0432.CCR-21-0164
Combining AFM13, a Bispecific CD30/CD16 Antibody, with Cytokine-Activated Blood and Cord Blood-Derived NK Cells Facilitates CAR-like Responses Against CD30+ Malignancies
Abstract
Purpose: Natural killer (NK)-cell recognition and function against NK-resistant cancers remain substantial barriers to the broad application of NK-cell immunotherapy. Potential solutions include bispecific engagers that target NK-cell activity via an NK-activating receptor when simultaneously targeting a tumor-specific antigen, as well as enhancing functionality using IL12/15/18 cytokine pre-activation.
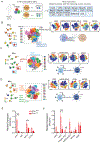
Experimental design: We assessed single-cell NK-cell responses stimulated by the tetravalent bispecific antibody AFM13 that binds CD30 on leukemia/lymphoma targets and CD16A on various types of NK cells using mass cytometry and cytotoxicity assays. The combination of AFM13 and IL12/15/18 pre-activation of blood and cord blood-derived NK cells was investigated in vitro and in vivo.
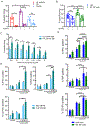
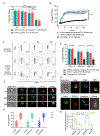
Results: We found heterogeneity within AFM13-directed conventional blood NK cell (cNK) responses, as well as consistent AFM13-directed polyfunctional activation of mature NK cells across donors. NK-cell source also impacted the AFM13 response, with cNK cells from healthy donors exhibiting superior responses to those from patients with Hodgkin lymphoma. IL12/15/18-induced memory-like NK cells from peripheral blood exhibited enhanced killing of CD30+ lymphoma targets directed by AFM13, compared with cNK cells. Cord-blood NK cells preactivated with IL12/15/18 and ex vivo expanded with K562-based feeders also exhibited enhanced killing with AFM13 stimulation via upregulation of signaling pathways related to NK-cell effector function. AFM13-NK complex cells exhibited enhanced responses to CD30+ lymphomas in vitro and in vivo.
Conclusions: We identify AFM13 as a promising combination with cytokine-activated adult blood or cord-blood NK cells to treat CD30+ hematologic malignancies, warranting clinical trials with these novel combinations.
©2021 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflict of Interest:
TAF and MMBE are consultants and have equity interest in Wugen, and may receive royalty income based on a technology developed by TAF and MMBE and licensed by Washington University to Wugen. JK and WF are employees of Affimed. MT is an employee of Arjuna Therapeutics. TAF has received research support from ImmunityBio, Compass Therapeutics, and HCW Biologics, and advises Kiadis, Nkarta, Indapta, and Orca Biosystems. LNK, RB, EL, SOA, EJS, KR and The University of Texas MD Anderson Cancer Center has an institutional financial conflict of interest with Affimed GmbH. This institutional financial conflict of interest is related to the research reported in this publication. Because MD Anderson is committed to the protection of human subjects and the effective management of its financial conflicts of interest in relation to its research activities, MD Anderson is implementing an Institutional Conflict of Interest Management and Monitoring Plan to manage and monitor the conflict of interest with respect to MD Anderson’s conduct of any other ongoing or future research related to this relationship. LNK, PPB, RB, MD, EL, SOA, REC, EJS, KR and The University of Texas MD Anderson Cancer Center have institutional financial conflict of interest with Takeda Pharmaceutical for the licensing of the technology related to CAR-NK cell research. MD Anderson has implemented an Institutional Conflict of Interest Management and Monitoring Plan to manage and monitor the conflict of interest with respect to MDACC’s conduct of any other ongoing or future research related to this relationship. KR participates on Scientific Advisory Board for GemoAb, AvengeBio, Virogin, GSK and Bayer. The remaining authors declare no competing financial interests.
Figures



References
-
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018. page 1069–86. - PubMed
-
- Garrido F, Cabrera T, Concha A, Glew S, Ruiz-Cabello F, Stern PL. Natural history of HLA expression during tumour development. Immunol. Today 1993. [Accessed 05/05/2020]. page 491–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

