Eliapixant (BAY 1817080), a P2X3 receptor antagonist, in refractory chronic cough: a randomised, placebo-controlled, crossover phase 2a study
- PMID: 33986030
- PMCID: PMC8607926
- DOI: 10.1183/13993003.04240-2020
Eliapixant (BAY 1817080), a P2X3 receptor antagonist, in refractory chronic cough: a randomised, placebo-controlled, crossover phase 2a study
Abstract
Background: ATP acting via P2X3 receptors is an important mediator of refractory chronic cough (RCC). This phase 2a double-blinded crossover study assessed the safety, tolerability and efficacy of eliapixant (BAY 1817080), a selective P2X3 receptor antagonist, in adults with RCC attending specialist centres.
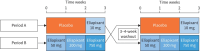
Methods: In period A, patients received placebo for 2 weeks then eliapixant 10 mg for 1 week. In period B, patients received eliapixant 50, 200 and 750 mg twice daily for 1 week per dose level. Patients were randomised 1:1 to period A-B (n=20) or B-A (n=20). The primary efficacy end-point was change in cough frequency assessed over 24 h. The primary safety end-point was frequency and severity of adverse events (AEs).
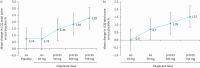
Results: 37 patients completed randomised therapy. Mean cough frequency fell by 17.4% versus baseline with placebo. Eliapixant reduced cough frequency at doses ≥50 mg (reduction versus placebo at 750 mg: 25% (90% CI 11.5-36.5%); p=0.002). Doses ≥50 mg also significantly reduced cough severity. AEs, mostly mild or moderate, were reported in 65% of patients with placebo and 41-49% receiving eliapixant. Cumulative rates of taste-related AEs were 3% with placebo and 5-21% with eliapixant; all were mild.
Conclusions: Selective P2X3 antagonism with eliapixant significantly reduced cough frequency and severity, confirming this as a viable therapeutic pathway for RCC. Taste-related side-effects were lower at therapeutic doses than with the less selective P2X3 antagonist gefapixant. Selective P2X3 antagonism appears to be a novel therapeutic approach for RCC.
Trial registration: ClinicalTrials.gov NCT03310645.
Copyright ©The authors 2021.
Conflict of interest statement
Conflict of interest: A. Morice reports grants, personal fees, nonfinancial support and other from Bayer AG and Bayer US, during the course of the study; personal fees, nonfinancial support and other from Bellus Health and Merck Sharp & Dohme Corp., personal fees and nonfinancial support from AstraZeneca, Chiesi Ltd and Boehringer Ingelheim, grants, personal fees, nonfinancial support and other from Sanofi, grants, personal fees and nonfinancial support from GlaxoSmithKline, Respivant Sciences, Inc. and Philips Respironics, grants, personal fees and other from NeRRe Therapeutics, grants from Menio Therapeutics, outside the submitted work. Conflict of interest: J.A. Smith reports grants and personal fees from Bayer AG, during the course of the study; grants and personal fees from Bellus Health, Shionogi Inc. and Merck Inc., outside the submitted work; and the VitaloJAK algorithm has been licensed by Manchester University NHS Foundation Trust and the University of Manchester to Vitalograph Ltd and Vitalograph Ireland (Ltd); Manchester University NHS Foundation Trust receives royalties which may be shared with the clinical division in which J.A. Smith works. Conflict of interest: L. McGarvey reports grants and personal fees from Bayer AG, during the conduct of the study; grants, personal fees and nonfinancial support from Chiesi, grants and personal fees from Merck & Co., Inc. and Bellus Health, nonfinancial support from Boehringer Ingelheim, personal fees from Applied Clinical Intelligence, Shionogi Inc., GlaxoSmithKline, NeRRe Therapeutics and Nocion Therapeutics, other from AstraZeneca, outside the submitted work. Conflict of interest: S.S. Birring reports grants and personal fees from Merck, personal fees from Bayer, Shionogi Inc., Bellus Health, NeRRe Therapeutics, Nocion Therapeutics, Boehringer Ingelheim and GlaxoSmithKline, outside the submitted work. Conflict of interest: S.M. Parker reports personal fees for consultancy from Menlo and Merck, outside the submitted work. Conflict of interest: A. Turner has nothing to disclose. Conflict of interest: T. Hummel reports grants from Sony, Smell and Taste Lab, Takasago and Aspuraclip, personal fees from Frequency Therapeutics and Baiafoods, outside the submitted work. Conflict of interest: I. Gashaw was an employee of Bayer AG when the study was designed and conducted but is now an employee of Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim, Germany. Conflict of interest: L. Fels is an employee of Bayer AG. Conflict of interest: S. Klein is an employee of Bayer AG. Conflict of interest: K. Franke is an employee of Bayer AG. Conflict of interest: C. Friedrich is an employee of Bayer AG.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
