Acute Respiratory Illnesses in Children in the SARS-CoV-2 Pandemic: Prospective Multicenter Study
- PMID: 33986150
- PMCID: PMC8338906
- DOI: 10.1542/peds.2021-051462
Acute Respiratory Illnesses in Children in the SARS-CoV-2 Pandemic: Prospective Multicenter Study
Abstract
Objectives: Nonpharmaceutical interventions against coronavirus disease 2019 likely have a role in decreasing viral acute respiratory illnesses (ARIs). We aimed to assess the frequency of respiratory syncytial virus (RSV) and influenza ARIs before and during the coronavirus disease 2019 pandemic.
Methods: This study was a prospective, multicenter, population-based ARI surveillance, including children seen in the emergency departments and inpatient settings in 7 US cities for ARI. Respiratory samples were collected and evaluated by molecular testing. Generalized linear mixed-effects models were used to evaluate the association between community mitigation and number of eligible and proportion of RSV and influenza cases.
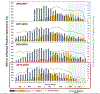
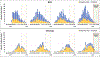
Results: Overall, 45 759 children were eligible; 25 415 were enrolled and tested; 25% and 14% were RSV-positive and influenza-positive, respectively. In 2020, we noted a decrease in eligible and enrolled ARI subjects after community mitigation measures were introduced, with no RSV or influenza detection from April 5, 2020, to April 30, 2020. Compared with 2016-2019, there was an average of 10.6 fewer eligible ARI cases per week per site and 63.9% and 45.8% lower odds of patients testing positive for RSV and influenza, respectively, during the 2020 community mitigation period. In all sites except Seattle, the proportions of positive tests for RSV and influenza in the 2020 community mitigation period were lower than predicted.
Conclusions: Between March and April 2020, rapid declines in ARI cases and the proportions of RSV and influenza in children were consistently noted across 7 US cities, which could be attributable to community mitigation measures against severe acute respiratory syndrome coronavirus 2.
Copyright © 2021 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: Dr Schuster receives support from Merck; Dr Williams is on boards for Quidel and GlaxoSmithKline; Dr Harrison’s institution receives support from GlaxoSmithKline, Merck, and Pfizer; Dr Englund is a consultant for Sanofi Pasteur and Meissa Vaccines and receives institutional research support from AstraZeneca, GlaxoSmithKline, Pfizer, and Novavax; Dr Halasa has grant funding from Sanofi and Quidel and received an honorarium from an educational grant from Genentech; the other authors have indicated they have no potential conflicts of interest to disclose.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

