Enantioselective access to chiral aliphatic amines and alcohols via Ni-catalyzed hydroalkylations
- PMID: 33986269
- PMCID: PMC8119980
- DOI: 10.1038/s41467-021-22983-7
Enantioselective access to chiral aliphatic amines and alcohols via Ni-catalyzed hydroalkylations
Abstract
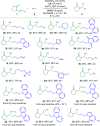
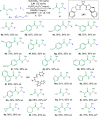
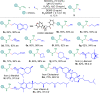
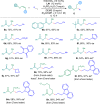
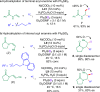
Chiral aliphatic amine and alcohol derivatives are ubiquitous in pharmaceuticals, pesticides, natural products and fine chemicals, yet difficult to access due to the challenge to differentiate between the spatially and electronically similar alkyl groups. Herein, we report a nickel-catalyzed enantioselective hydroalkylation of acyl enamines and enol esters with alkyl halides to afford enantioenriched α-branched aliphatic acyl amines and esters in good yields with excellent levels of enantioselectivity. The operationally simple protocol provides a straightforward access to chiral secondary alkyl-substituted amine and secondary alkyl-substituted alcohol derivatives from simple starting materials with great functional group tolerance.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Nugent TC, El-Shazly M. Chiral amine synthesis – recent developments and trends for enamide reduction, reductive amination, and imine reduction. Adv. Synth. Catal. 2010;352:753–819. doi: 10.1002/adsc.200900719. - DOI
-
- McGrath NA, Brichacek M, Njardarson JT. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 2010;87:1348–1349. doi: 10.1021/ed1003806. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

