Highly conserved, non-human-like, and cross-reactive SARS-CoV-2 T cell epitopes for COVID-19 vaccine design and validation
- PMID: 33986292
- PMCID: PMC8119491
- DOI: 10.1038/s41541-021-00331-6
Highly conserved, non-human-like, and cross-reactive SARS-CoV-2 T cell epitopes for COVID-19 vaccine design and validation
Abstract
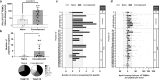
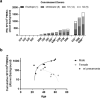
Natural and vaccine-induced SARS-CoV-2 immunity in humans has been described but correlates of protection are not yet defined. T cells support the SARS-CoV-2 antibody response, clear virus-infected cells, and may be required to block transmission. In this study, we identified peptide epitopes associated with SARS-CoV-2 T-cell immunity. Using immunoinformatic methods, T-cell epitopes from spike, membrane, and envelope were selected for maximal HLA-binding potential, coverage of HLA diversity, coverage of circulating virus, and minimal potential cross-reactivity with self. Direct restimulation of PBMCs collected from SARS-CoV-2 convalescents confirmed 66% of predicted epitopes, whereas only 9% were confirmed in naive individuals. However, following a brief period of epitope-specific T-cell expansion, both cohorts demonstrated robust T-cell responses to 97% of epitopes. HLA-DR3 transgenic mouse immunization with peptides co-formulated with poly-ICLC generated a potent Th1-skewed, epitope-specific memory response, alleviating safety concerns of enhanced respiratory disease associated with Th2 induction. Taken together, these epitopes may be used to improve our understanding of natural and vaccine-induced immunity, and to facilitate the development of T-cell-targeted vaccines that harness pre-existing SARS-CoV-2 immunity.
Conflict of interest statement
A.S.D.G. and W.D.M. are senior officers and shareholders, and L.M.M., A.H.G., C.M.B., F.T., B.G.M., and L.M. are employees of EpiVax, Inc., a privately owned biotechnology company located in Providence, RI. M.F.P. is an employee of EpiVax Therapeutics, Inc. Both EpiVax and EpiVax Therapeutics are developing COVID-19 vaccines. These authors acknowledge that there is a potential conflict of interest related to their relationship with EpiVax and attest that the work contained in this research report is free of any bias that might be associated with the commercial goals of the company. A.S. is a senior officer at Oncovir, Inc. Oncovir is developing a COVID-19 therapy. He acknowledges that there is a potential conflict of interest related to his relationship with Oncovir and attests that the work contained in this research report is free of any bias that might be associated with the commercial goals of the company.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

