Type I Interferons in Systemic Autoimmune Diseases: Distinguishing Between Afferent and Efferent Functions for Precision Medicine and Individualized Treatment
- PMID: 33986670
- PMCID: PMC8112244
- DOI: 10.3389/fphar.2021.633821
Type I Interferons in Systemic Autoimmune Diseases: Distinguishing Between Afferent and Efferent Functions for Precision Medicine and Individualized Treatment
Abstract
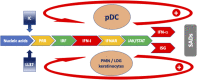
A sustained increase in type I interferon (IFN-I) may accompany clinical manifestations and disease activity in systemic autoimmune diseases (SADs). Despite the very frequent presence of IFN-I in SADs, clinical manifestations are extremely varied between and within SADs. The present short review will address the following key questions associated with high IFN-I in SADs in the perspective of precision medicine. 1) What are the mechanisms leading to high IFN-I? 2) What are the predisposing conditions favoring high IFN-I production? 3) What is the role of IFN-I in the development of distinct clinical manifestations within SADs? 4) Would therapeutic strategies targeting IFN-I be helpful in controlling or even preventing SADs? In answering these questions, we will underlie areas of incertitude and the intertwined role of autoantibodies, immune complexes, and neutrophils.
Keywords: autoantibody (autoAb); genetic polymorphism; interferon; interferon-stimulated genes (ISGs); keratinocytes; polymorphonuclear neutrophils (PMN); systemic autoimmune diseases (SADs); systemic lupus erythematosus (SLE).
Copyright © 2021 Chasset, Dayer and Chizzolini.
Conflict of interest statement
FC was supported by a research travel grant from Institut Servier, Paris (France). JD declares no conflict of interest. CC received honoraria as an advisor or invited speaker from GSK, Roche, Boehringer Ingelheim.
Figures

Similar articles
-
SARS-Cov2 acute and post-active infection in the context of autoimmune and chronic inflammatory diseases.J Transl Autoimmun. 2022;5:100154. doi: 10.1016/j.jtauto.2022.100154. Epub 2022 Apr 12. J Transl Autoimmun. 2022. PMID: 35434592 Free PMC article.
-
Targeting type I interferons in systemic lupus erythematous.Front Pharmacol. 2023 Jan 16;13:1046687. doi: 10.3389/fphar.2022.1046687. eCollection 2022. Front Pharmacol. 2023. PMID: 36726783 Free PMC article. Review.
-
Identification of highly active systemic lupus erythematosus by combined type I interferon and neutrophil gene scores vs classical serologic markers.Rheumatology (Oxford). 2020 Nov 1;59(11):3468-3478. doi: 10.1093/rheumatology/keaa167. Rheumatology (Oxford). 2020. PMID: 32375176
-
High levels of circulating interferons type I, type II and type III associate with distinct clinical features of active systemic lupus erythematosus.Arthritis Res Ther. 2019 Apr 29;21(1):107. doi: 10.1186/s13075-019-1878-y. Arthritis Res Ther. 2019. PMID: 31036046 Free PMC article.
-
Signaling Pathways of Type I and Type III Interferons and Targeted Therapies in Systemic Lupus Erythematosus.Cells. 2019 Aug 23;8(9):963. doi: 10.3390/cells8090963. Cells. 2019. PMID: 31450787 Free PMC article. Review.
Cited by
-
Case report: IgG4-related intracranial lesions mimicking multiple sclerosis in a 14-year-old girl.Front Neurol. 2022 Sep 28;13:1007153. doi: 10.3389/fneur.2022.1007153. eCollection 2022. Front Neurol. 2022. PMID: 36247763 Free PMC article.
-
VRK1 promotes DNA-induced type I interferon production.Mol Biol Rep. 2024 Mar 27;51(1):453. doi: 10.1007/s11033-024-09414-8. Mol Biol Rep. 2024. PMID: 38536553
-
Why Do We Need JAK Inhibitors in Systemic Lupus Erythematosus?Int J Mol Sci. 2022 Oct 4;23(19):11788. doi: 10.3390/ijms231911788. Int J Mol Sci. 2022. PMID: 36233087 Free PMC article. Review.
-
Type III interferon exerts thymic stromal lymphopoietin in mediating adaptive antiviral immune response.Front Immunol. 2023 Sep 22;14:1250541. doi: 10.3389/fimmu.2023.1250541. eCollection 2023. Front Immunol. 2023. PMID: 37809098 Free PMC article. Review.
-
Targeting intracellular pathways in idiopathic inflammatory myopathies: A narrative review.Front Med (Lausanne). 2023 Mar 13;10:1158768. doi: 10.3389/fmed.2023.1158768. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36993798 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

