Microphysiological Systems for Studying Cellular Crosstalk During the Neutrophil Response to Infection
- PMID: 33986752
- PMCID: PMC8111168
- DOI: 10.3389/fimmu.2021.661537
Microphysiological Systems for Studying Cellular Crosstalk During the Neutrophil Response to Infection
Abstract
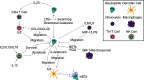
Neutrophils are the primary responders to infection, rapidly migrating to sites of inflammation and clearing pathogens through a variety of antimicrobial functions. This response is controlled by a complex network of signals produced by vascular cells, tissue resident cells, other immune cells, and the pathogen itself. Despite significant efforts to understand how these signals are integrated into the neutrophil response, we still do not have a complete picture of the mechanisms regulating this process. This is in part due to the inherent disadvantages of the most-used experimental systems: in vitro systems lack the complexity of the tissue microenvironment and animal models do not accurately capture the human immune response. Advanced microfluidic devices incorporating relevant tissue architectures, cell-cell interactions, and live pathogen sources have been developed to overcome these challenges. In this review, we will discuss the in vitro models currently being used to study the neutrophil response to infection, specifically in the context of cell-cell interactions, and provide an overview of their findings. We will also provide recommendations for the future direction of the field and what important aspects of the infectious microenvironment are missing from the current models.
Keywords: antimicrobial functions; cell-cell interactions; in vitro models; infection; inflammation; innate immunity; microfluidics; neutrophil.
Copyright © 2021 Richardson, Calo and Hind.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

