Perinatal DEHP exposure induces sex- and tissue-specific DNA methylation changes in both juvenile and adult mice
- PMID: 33986952
- PMCID: PMC8107644
- DOI: 10.1093/eep/dvab004
Perinatal DEHP exposure induces sex- and tissue-specific DNA methylation changes in both juvenile and adult mice
Abstract
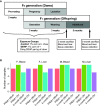
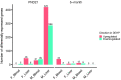
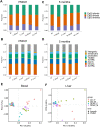
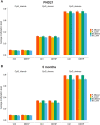
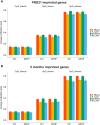
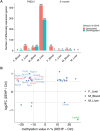
Di(2-ethylhexyl) phthalate (DEHP) is a type of phthalate plasticizer found in a variety of consumer products and poses a public health concern due to its metabolic and endocrine disruption activities. Dysregulation of epigenetic modifications, including DNA methylation, has been shown to be an important mechanism for the pathogenic effects of prenatal exposures, including phthalates. In this study, we used an established mouse model to study the effect of perinatal DEHP exposure on the DNA methylation profile in liver (a primary target tissue of DEHP) and blood (a common surrogate tissue) of both juvenile and adult mice. Despite exposure ceasing at 3 weeks of age (PND21), we identified thousands of sex-specific differential DNA methylation events in 5-month old mice, more than identified at PND21, both in blood and liver. Only a small number of these differentially methylated cytosines (DMCs) overlapped between the time points, or between tissues (i.e. liver and blood), indicating blood may not be an appropriate surrogate tissue to estimate the effects of DEHP exposure on liver DNA methylation. We detected sex-specific DMCs common between 3-week and 5-month samples, pointing to specific DNA methylation alterations that are consistent between weanling and adult mice. In summary, this is the first study to assess the genome-wide DNA methylation profiles in liver and blood at two different aged cohorts in response to perinatal DEHP exposure. Our findings cast light on the implications of using surrogate tissue instead of target tissue in human population-based studies and identify epigenetic biomarkers for DEHP exposure.
Keywords: DEHP exposure; DNA methylation; bisulfite sequencing; blood; liver; mouse model; perinatal.
© The Author(s) 2021. Published by Oxford University Press.
Figures
Similar articles
-
Effects of Developmental Lead and Phthalate Exposures on DNA Methylation in Adult Mouse Blood, Brain, and Liver Identifies Tissue- and Sex-Specific Changes with Implications for Genomic Imprinting.bioRxiv [Preprint]. 2023 Oct 10:2023.09.29.560131. doi: 10.1101/2023.09.29.560131. bioRxiv. 2023. Update in: Environ Health Perspect. 2024 Jun;132(6):67003. doi: 10.1289/EHP14074. PMID: 37873115 Free PMC article. Updated. Preprint.
-
Sex-Specific Programming of Cardiac DNA Methylation by Developmental Phthalate Exposure.Epigenet Insights. 2020 Aug 5;13:2516865720939971. doi: 10.1177/2516865720939971. eCollection 2020. Epigenet Insights. 2020. PMID: 32864567 Free PMC article.
-
Perinatal exposures to phthalates and phthalate mixtures result in sex-specific effects on body weight, organ weights and intracisternal A-particle (IAP) DNA methylation in weanling mice.J Dev Orig Health Dis. 2019 Apr;10(2):176-187. doi: 10.1017/S2040174418000430. Epub 2018 Jul 11. J Dev Orig Health Dis. 2019. PMID: 29991372 Free PMC article.
-
Review on Toxic Effects of Di(2-ethylhexyl) Phthalate on Zebrafish Embryos.Toxics. 2021 Aug 21;9(8):193. doi: 10.3390/toxics9080193. Toxics. 2021. PMID: 34437511 Free PMC article. Review.
-
A Review of Biomonitoring of Phthalate Exposures.Toxics. 2019 Apr 5;7(2):21. doi: 10.3390/toxics7020021. Toxics. 2019. PMID: 30959800 Free PMC article. Review.
Cited by
-
Impact of Endocrine Disruptors upon Non-Genetic Inheritance.Int J Mol Sci. 2022 Mar 20;23(6):3350. doi: 10.3390/ijms23063350. Int J Mol Sci. 2022. PMID: 35328771 Free PMC article. Review.
-
Preconception and developmental DEHP exposure alter liver metabolism in a sex-dependent manner in adult mouse offspring.Toxicology. 2023 Nov;499:153640. doi: 10.1016/j.tox.2023.153640. Epub 2023 Oct 6. Toxicology. 2023. PMID: 37806616 Free PMC article.
-
Effects of Prenatal Phthalate Exposure and Childhood Exercise on Maternal Behaviors in Female Rats at Postpartum: A Role of Oxtr Methylation in the Hypothalamus.Int J Mol Sci. 2021 Sep 12;22(18):9847. doi: 10.3390/ijms22189847. Int J Mol Sci. 2021. PMID: 34576011 Free PMC article.
-
Translational toxicoepigenetic Meta-Analyses identify homologous gene DNA methylation reprogramming following developmental phthalate and lead exposure in mouse and human offspring.Environ Int. 2024 Apr;186:108575. doi: 10.1016/j.envint.2024.108575. Epub 2024 Mar 11. Environ Int. 2024. PMID: 38507935 Free PMC article.
-
Effects of Developmental Lead and Phthalate Exposures on DNA Methylation in Adult Mouse Blood, Brain, and Liver: A Focus on Genomic Imprinting by Tissue and Sex.Environ Health Perspect. 2024 Jun;132(6):67003. doi: 10.1289/EHP14074. Epub 2024 Jun 4. Environ Health Perspect. 2024. PMID: 38833407 Free PMC article.
References
-
- ATSDR. Toxicological profile: di(2-ethylhexyl)phthalate (DEHP). Agency for Toxic Substances and Disease Registry 2002. https://www.atsdr.cdc.gov/toxprofiles/tp9.pdf. Last accessed on March 30th 221 - PubMed
-
- Bergé A, Cladière M, Gasperi J, Coursimault A, Tassin B, Moilleron R. Meta-analysis of environmental contamination by phthalates. Environ Sci Pollut Res 2013. - PubMed
-
- Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P et al. NTP center for the evaluation of risks to human reproduction: phthalates expert panel report on the reproductive and developmental toxicity of butyl benzyl phthalate. Reprod Toxicol 2002. - PubMed
-
- Högberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, Filipsson AF, Jansson B, Johansson N, Appelgren M, Håkansson H. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect 2008;116:334–9. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

