Lactobacillus reuteri TSR332 and Lactobacillus fermentum TSF331 stabilize serum uric acid levels and prevent hyperuricemia in rats
- PMID: 33986988
- PMCID: PMC8101448
- DOI: 10.7717/peerj.11209
Lactobacillus reuteri TSR332 and Lactobacillus fermentum TSF331 stabilize serum uric acid levels and prevent hyperuricemia in rats
Abstract
Background: Uric acid (UA) is the end product of purine metabolism in the liver and is excreted by the kidneys. When purine metabolism is impaired, the serum UA level will be elevated (hyperuricemia) and eventually lead to gout. During evolution, humans and some primates have lost the gene encoding uricase, which is vital in UA metabolism. With the advances of human society, the prevalence of hyperuricemia has dramatically increased because of the refined food culture. Hyperuricemia can be controlled by drugs, such as allopurinol and probenecid. However, these drugs have no preventive effect and are associated with unpleasant side effects. An increasing number of probiotic strains, which are able to regulate host metabolism and prevent chronic diseases without harmful side effects, have been characterized. The identification of probiotic strains, which are able to exert beneficial effects on UA metabolism, will provide an alternative healthcare strategy for patients with hyperuricemia, especially for those who are allergic to anti-hyperuricemia drugs.
Methods: To elicit hyperuricemia, rats in the symptom control group (HP) were injected with potassium oxonate and fed a high-purine diet. Rats in the probiotic groups received the high-purine diet, oxonate injection, and supplements of probiotic strains TSR332, TSF331, or La322. Rats in the blank control group (C) received a standard diet (AIN-93G) and oxonate injection.
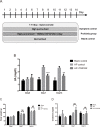
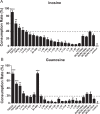
Results: Purine-utilizing strains of probiotics were screened using high-pressure liquid chromatography (HPLC) in vitro, and the lowering effect on serum UA levels was analyzed in hyperuricemia rats in vivo. We found that Lactobacillus reuteri strain TSR332 and Lactobacillus fermentum strain TSF331 displayed significantly strong assimilation of inosine (90%; p = 0.00003 and 59%; p = 0.00545, respectively) and guanosine (78%; p = 0.00012 and 51%; p = 0.00062, respectively) within 30 min in vitro. Further animal studies revealed that serum UA levels were significantly reduced by 60% (p = 0.00169) and 30% (p = 0.00912), respectively, in hyperuricemic rats treated with TSR332 and TSF331 for 8 days. Remarkably, TSR332 ameliorated the occurrence of hyperuricemia, and no evident side effects were observed. Overall, our study indicates that TSR332 and TSF331 are potential functional probiotic strains for controlling the development of hyperuricemia.
Keywords: Gout; Hyperuricemia; Lactobacillus; Probiotics; Purine metabolism.
©2021 Kuo et al.
Conflict of interest statement
Yi-Wei Kuo, Shih-Hung Hsieh, Jui-Fen Chen, Cheng-Ruei Liu, Ching-Wei Chen, Yu-Fen Huang, and Hsieh-Hsun Ho are employed by Glac Biotech Co., Ltd.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

