The hypoferremic response to acute inflammation is maintained in thalassemia mice even under parenteral iron loading
- PMID: 33987030
- PMCID: PMC8092106
- DOI: 10.7717/peerj.11367
The hypoferremic response to acute inflammation is maintained in thalassemia mice even under parenteral iron loading
Abstract
Background: Hepcidin controls iron homeostasis by inducing the degradation of the iron efflux protein, ferroportin (FPN1), and subsequently reducing serum iron levels. Hepcidin expression is influenced by multiple factors, including iron stores, ineffective erythropoiesis, and inflammation. However, the interactions between these factors under thalassemic condition remain unclear. This study aimed to determine the hypoferremic and transcriptional responses of iron homeostasis to acute inflammatory induction by lipopolysaccharide (LPS) in thalassemic (Hbbth3 /+) mice with/without parenteral iron loading with iron dextran.
Methods: Wild type and Hbbth3 /+ mice were intramuscularly injected with 5 mg of iron dextran once daily for two consecutive days. After a 2-week equilibration, acute inflammation was induced by an intraperitoneal injection of a single dose of 1 µg/g body weight of LPS. Control groups for both iron loading and acute inflammation received equal volume(s) of saline solution. Blood and tissue samples were collected at 6 hours after LPS (or saline) injection. Iron parameters and mRNA expression of hepcidin as well as genes involved in iron transport and metabolism in wild type and Hbbth3 /+ mice were analyzed and compared by Kruskal-Wallis test with pairwise Mann-Whitney U test.
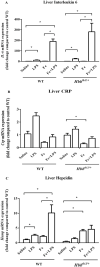
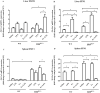
Results: We found the inductive effects of LPS on liver IL-6 mRNA expression to be more pronounced under parenteral iron loading. Upon LPS administration, splenic erythroferrone (ERFE) mRNA levels were reduced only in iron-treated mice, whereas, liver bone morphogenetic protein 6 (BMP6) mRNA levels were decreased under both control and parenteral iron loading conditions. Despite the altered expression of the aforementioned hepcidin regulators, the stimulatory effect of LPS on hepcidin mRNA expression was blunt in iron-treated Hbbth3 /+ mice. Contrary to the blunted hepcidin response, LPS treatment suppressed FPN1 mRNA expression in the liver, spleen, and duodenum, as well as reduced serum iron levels of Hbbth3 /+ mice with parenteral iron loading.
Conclusion: Our study suggests that a hypoferremic response to LPS-induced acute inflammation is maintained in thalassemic mice with parenteral iron loading in a hepcidin-independent manner.
Keywords: Hepcidin; Iron loading; Iron transporters; Lipopolysaccharide; Thalassemic mice.
© 2021 Sanyear et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

