Innovative Approaches in the Battle Against Cancer Recurrence: Novel Strategies to Combat Dormant Disseminated Tumor Cells
- PMID: 33987095
- PMCID: PMC8111294
- DOI: 10.3389/fonc.2021.659963
Innovative Approaches in the Battle Against Cancer Recurrence: Novel Strategies to Combat Dormant Disseminated Tumor Cells
Abstract
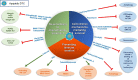
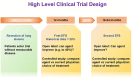
Cancer recurrence remains a great fear for many cancer survivors following their initial, apparently successful, therapy. Despite significant improvement in the overall survival of many types of cancer, metastasis accounts for ~90% of all cancer mortality. There is a growing understanding that future therapeutic practices must accommodate this unmet medical need in preventing metastatic recurrence. Accumulating evidence supports dormant disseminated tumor cells (DTCs) as a source of cancer recurrence and recognizes the need for novel strategies to target these tumor cells. This review presents strategies to target dormant quiescent DTCs that reside at secondary sites. These strategies aim to prevent recurrence by maintaining dormant DTCs at bay, or eradicating them. Various approaches are presented, including: reinforcing the niche where dormant DTCs reside in order to keep dormant DTCs at bay; promoting cell intrinsic mechanisms to induce dormancy; preventing the engagement of dormant DTCs with their supportive niche in order to prevent their reactivation; targeting cell-intrinsic mechanisms mediating long-term survival of dormant DTCs; sensitizing dormant DTCs to chemotherapy treatments; and, inhibiting the immune evasion of dormant DTCs, leading to their demise. Various therapeutic approaches, some of which utilize drugs that are already approved, or have been tested in clinical trials and may be considered for repurposing, will be discussed. In addition, clinical evidence for the presence of dormant DTCs will be reviewed, along with potential prognostic biomarkers to enable the identification and stratification of patients who are at high risk of recurrence, and who could benefit from novel dormant DTCs targeting therapies. Finally, we will address the shortcomings of current trial designs for determining activity against dormant DTCs and provide novel approaches.
Keywords: cancer recurrence; disseminated tumor cells; immune evasion and clinical trials; metastasis; tumor dormancy; tumor microenvironment.
Copyright © 2021 Sauer, Reed, Ihnat, Hurst, Warshawsky and Barkan.
Conflict of interest statement
SS, RH and DW were employed by Vuja De Sciences Inc. DB and MI have consulting agreements with Vuja De Sciences, Inc. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Medical

