miR-1301-3p Promotes Cell Proliferation and Facilitates Cell Cycle Progression via Targeting SIRT1 in Gastric Cancer
- PMID: 33987098
- PMCID: PMC8112236
- DOI: 10.3389/fonc.2021.664242
miR-1301-3p Promotes Cell Proliferation and Facilitates Cell Cycle Progression via Targeting SIRT1 in Gastric Cancer
Abstract
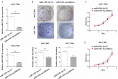
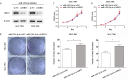
So far, many existing evidences indicate that microRNAs (miRNA) are closely associated with the tumorigenesis and progression of various tumors. It has been reported that miR-1301-3p is abnormally expressed in several malignant tumors. However, the role of miR-1301-3p in gastric cancer (GC) remains unclear and is worth studying. Through qRT-PCR, the expression of miR-1301-3p and SIRT1 were detected in GC tissues and cells. The cell proliferation and cell cycle were measured through CCK-8 assay and clone formation assay. Dual luciferase reporter assay was used to determine the target of miR-1301-3p. Though tumorigenesis assay, we monitored the effect of miR-1301-3p on GC cell growth in vivo. miR-1301-3p was upregulated in GC tissues and cells in our study. Overexpression of miR-1301-3p accelerated GC cell proliferation, cell cycle progression and tumorigenesis. Notably, altering the expression miR-1301-3p caused deregulation of Cyclin D1, CDK4, c-Myc and P21. Furthermore, SIRT1 was the direct target of miR-1301-3p by luciferase reporter assay. After transfecting with miR-1301-3p inhibitor, we found that knockdown of SIRT1 could enhance the ability of proliferation. Our results identify miR-1301-3p as a novel potential therapeutic target that is associated with the tumorigenesis and progression of gastric cancer.
Keywords: SIRT1; cell cycle; cell proliferation; gastric cancer; miR-1301-3p.
Copyright © 2021 Luo, Fan, Ma, Yang, He, Ge, Jiang, Xu and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK. Mirna Deregulation in Cancer Cells and the Tumor Microenvironment. Cancer Discovery (2016) 6(3):235–46. 10.1158/2159-8290.CD-15-0893 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

