Cost-Effectiveness Analysis of Atezolizumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer With Different PD-L1 Expression Status
- PMID: 33987103
- PMCID: PMC8111076
- DOI: 10.3389/fonc.2021.669195
Cost-Effectiveness Analysis of Atezolizumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer With Different PD-L1 Expression Status
Abstract
Background: Atezolizumab could significantly improve clinical outcomes and was associated with less toxicity compared with chemotherapy as the first-line treatment of PD-L1-selected patients with EGFR and ALK wild-type metastatic non-small-cell lung cancer (NSCLC). However, the economic outcomes remain unclear yet in China. This study aimed to investigate the cost-effectiveness of atezolizumab versus chemotherapy as first-line therapy for metastatic NSCLC with different PD-L1 expression status from the Chinese health sector perspective.
Methods: A decision-analytic model was conducted to evaluate the economic outcomes for the first-line treatment of EGFR and ALK wild-type metastatic NSCLC with atezolizumab and chemotherapy in high PD-L1 expression, high or intermediate PD-L1 expression and any PD-L1 expression populations, respectively. The efficacy and safety data were obtained from the IMpower110 trial. Cost and utility values were gathered from the local charges and published literatures. Incremental cost-effectiveness ratio (ICER) was estimated. A scenario analysis for a patient assistance program (PAP) was conducted. One-way and probabilistic sensitivity analyses were performed to explore the robustness of the model results.
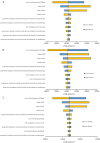
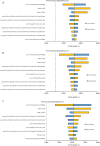
Results: Atezolizumab yielded additional 0.91 QALYs, 0.57 QALYs, 0.42 QALYs in comparison with chemotherapy, and the ICERs were $123,778.60/QALY, $142,827.19/QALY, $168,902.66/QALY in the high PD-L1 expression, high or intermediate PD-L1 expression, and any PD-L1 expression populations, respectively. When PAP was available, the ICERs were $52,414.63/QALY, $52,329.73/QALY, $61,189.66/QALY in the three categories of PD-L1 expression status populations, respectively. The ICERs were exceed the willingness-to-pay (WTP) threshold of $30,828/QALY (three times of per capita gross domestic product of China in 2019) in China. One-way sensitivity analyses suggested that the cost of atezolizumab played a vital role in the model outcomes, and the probabilistic sensitivity analyses showed atezolizumab was unlikely to be cost-effective at the WTP threshold regardless of PD-L1 expression status and whether the PAP was available or not.
Conclusions: Atezolizumab as first-line treatment for PD-L1-selected metastatic NSCLC patients without EGFR mutations or ALK translocations is unlikely to be cost-effective compared with chemotherapy regardless of PD-L1 expression status in the Chinese context.
Keywords: atezolizumab; chemotherapy; cost-effectiveness; first-line treatment; non-small-cell lung cancer.
Copyright © 2021 Liu, Kang, Wang and Shang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- GBD 2017 DALYs. HALE Collaborators . Global, Regional, and National Disability-Adjusted Life-Years (Dalys) for 359 Diseases and Injuries and Healthy Life Expectancy (HALE) for 195 Countries and Territories, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet (2018) 392:1859–922. 10.1016/S0140-6736(18)32335-3 - DOI - PMC - PubMed
-
- Global Burden of Disease Cancer Collaboration . Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol (2018) 4:1553–68. 10.1001/jamaoncol.2018.2706 - DOI - PMC - PubMed
-
- Cai Y, Hu BH, Zhou GW. Analysis of Direct Economic Burden and Average Hospitalization Cost of Lung Cancer in China in 2011–2015. Chin Health Statistics (2018) 35:334–7. CNKISUNZGWT.0.2018-03-003
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

