Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis
- PMID: 33987952
- PMCID: PMC8417863
- DOI: 10.1002/onco.13824
Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis
Abstract
Background: Total neoadjuvant therapy (TNT) is a novel approach for locally advanced rectal cancer (LARC), which attempts to deliver both systemic chemotherapy and neoadjuvant chemoradiotherapy prior to surgery. However, its efficacy and safety remain controversial in randomized controlled trials (RCTs). We conducted this meta-analysis to assess such concerns.
Materials and methods: Head-to-head phase II/III RCTs were searched in Embase, PubMed, Web of Science, and the Cochrane Library, as well as other sources. The primary endpoint was pathologic complete response (pCR). Secondary endpoints were disease-free survival (DFS), overall survival (OS), local recurrence-free survival, distant metastasis-free survival, and the R0 resection rate.
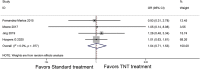
Results: Eight phase II/III RCTs involving 2,196 patients with LARC were assessed. The primary analysis demonstrated a statistically significant improvement in the pCR rate for TNT treatment (odds ratio, 1.77; 95% confidence interval [CI], 1.28-2.45; p = .0005). TNT treatment also showed improvements in DFS and OS outcomes compared with standard chemoradiotherapy (hazard ratio [HR], 0.83; 95% CI, 0.72-0.96; p = .03 and HR, 0.88; 95% CI, 0.74-1.05; p = .15). In addition, TNT treatment showed significant efficacy in reducing the risk of distant metastasis (HR, 0.81; 95% CI, 0.68-0.95; p = .012).
Conclusion: The overall pCR rate may be improved with TNT compared with standard treatment. The TNT strategy may also improve DFS and OS and reduce the risk of distant metastasis.
Implications for practice: Locally advanced rectal cancer (LARC) is a relatively common disease, with a poor prognosis because of its high metastatic potential. The role of total neoadjuvant therapy (TNT) has always been controversial. This meta-analysis found that TNT in LARC is associated with a significant improvement in overall pathologic complete response rate, disease-free survival, overall survival, and distant metastasis-free survival compared with standard treatment. TNT is a promising strategy for LARC, especially for patients who have little desire for surgery.
Keywords: Locally advanced rectal cancer (LARC); Meta-analysis; Pathologic complete response (pCR); Total neoadjuvant therapy (TNT).
© 2021 AlphaMed Press.
Conflict of interest statement
Figures


References
-
- Siegel RL, Miller KD, Fedewa SA et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177–193. - PubMed
-
- Glynne‐Jones R, Wyrwicz L, Tiret E et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017;28:iv22–iv40. - PubMed
-
- Ludmir EB, Palta M, Willett CG et al. Total neoadjuvant therapy for rectal cancer: An emerging option. Cancer 2017;123:1497–1506. - PubMed
-
- Nahas SC, Rizkallah Nahas CS, Sparapan Marques CF et al. Pathologic complete response in rectal cancer: Can we detect it? Lessons learned from a proposed randomized trial of watch‐and‐wait treatment of rectal cancer. Dis Colon Rectum 2016;59:255–263. - PubMed
-
- Rodel C, Liersch T, Becker H et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: Initial results of the German CAO/ARO/AIO‐04 randomised phase 3 trial. Lancet Oncol 2012; 13:679–687. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

