Relevance of treatment-free remission recommendations in chronic phase chronic leukemia patients treated with frontline tyrosine kinase inhibitors
- PMID: 33988316
- PMCID: PMC8178499
- DOI: 10.1002/cam4.3921
Relevance of treatment-free remission recommendations in chronic phase chronic leukemia patients treated with frontline tyrosine kinase inhibitors
Abstract
Background: Tyrosine kinase inhibitors (TKI) can be safely discontinued in chronic phase chronic myeloid leukemia (CP-CML) patients who had achieved a sustained deep molecular response. Based on the results of discontinuation trials, recommendations regarding patient selection for a treatment-free remission (TFR) attempt had been proposed. The aims of this study were to evaluate the rate of patients eligible for TKI discontinuation and molecular recurrence-free survival (MRFS) after stop according to recommendations.
Methods: Over a 10-year period, newly diagnosed CP-CML patients and treated with first-line TKI in the nine French participating centers were included. Eligibility to treatment discontinuation and MRFS were analyzed and compared according to selection criteria defined by recommendations and first-line treatments.
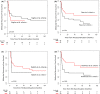
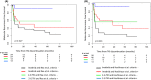
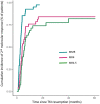
Results: From January 2006 to December 2015, 398 patients were considered. Among them, 73% and 27% of patients received imatinib or either second or third generation tyrosine kinase inhibitors as frontline treatment, respectively. Considering the selection criteria defined by recommendations, up to 55% of the patients were selected as optimal candidates for treatment discontinuation. Overall 95/398 (24%) discontinued treatment. MRFS was 51.8% [95% CI 41.41-62.19] at 2 years and 43.8% [31.45-56.15] at 5 years. Patients receiving frontline second-generation TKI and fulfilling the eligibility criteria suggested by recommendations had the lowest probability of molecular relapse after TKI stop when compare to others.
Conclusion: One third of CP-CML patients treated with TKI frontline fulfilled the selection criteria suggested by European LeukemiaNet TFR recommendations. Meeting selection criteria and second-generation TKI frontline were associated with the highest MRFS.
Keywords: molecular recurrence-free survival; recommendations; tyrosine kinase inhibitor discontinuation.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
GE is a consultant for Novartis, Bristol Myers Squibb, Pfizer, and Incyte Pharma, and has given some lectures for Bristol Myers Squibb, Incyte Pharma, Pfizer, and Novartis. He has received research grants from Novartis and Bristoll Myers Squibb. SD has given some lectures for Novartis and Incyte Pharma. FXM is a consultant for Novartis and Bristol Myers Squibb, and has given some lectures for Bristoll Myers Squibb, Incyte Pharma, Novartis, and Pfizer. He holds a research grant from Novartis. FBa, DA, FL, CD, CL, AS, EK, SM, MPF, FBi, MM, BT, FR, FD, CF, LV, SK, ACN, and AL have nothing to disclose. The authors declare no conflicts of interest for the current work.
Figures
References
-
- Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029‐1035. - PubMed
-
- Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122:515‐522. - PubMed
-
- Dulucq S, Astrugue C, Etienne G, Mahon FX, Benard A. Risk of molecular recurrence after tyrosine kinase inhibitor discontinuation in chronic myeloid leukaemia patients: a systematic review of literature with a meta‐analysis of studies over the last ten years. Br J Haematol. 2020;189:452‐468. - PubMed
-
- Rousselot P, Charbonnier A, Cony‐Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic‐phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32:424‐430. - PubMed
-
- Alfayez M, Richard‐Carpentier G, Jabbour E, et al. Sudden blastic transformation in treatment‐free remission chronic myeloid leukaemia. Br J Haematol. 2019;187:543‐545. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

