A TORC1-histone axis regulates chromatin organisation and non-canonical induction of autophagy to ameliorate ageing
- PMID: 33988501
- PMCID: PMC8186904
- DOI: 10.7554/eLife.62233
A TORC1-histone axis regulates chromatin organisation and non-canonical induction of autophagy to ameliorate ageing
Abstract
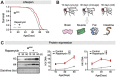
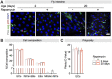
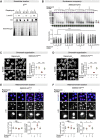
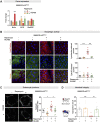
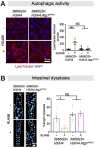
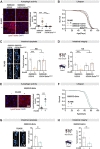
Age-related changes to histone levels are seen in many species. However, it is unclear whether changes to histone expression could be exploited to ameliorate the effects of ageing in multicellular organisms. Here we show that inhibition of mTORC1 by the lifespan-extending drug rapamycin increases expression of histones H3 and H4 post-transcriptionally through eIF3-mediated translation. Elevated expression of H3/H4 in intestinal enterocytes in Drosophila alters chromatin organisation, induces intestinal autophagy through transcriptional regulation, and prevents age-related decline in the intestine. Importantly, it also mediates rapamycin-induced longevity and intestinal health. Histones H3/H4 regulate expression of an autophagy cargo adaptor Bchs (WDFY3 in mammals), increased expression of which in enterocytes mediates increased H3/H4-dependent healthy longevity. In mice, rapamycin treatment increases expression of histone proteins and Wdfy3 transcription, and alters chromatin organisation in the small intestine, suggesting that the mTORC1-histone axis is at least partially conserved in mammals and may offer new targets for anti-ageing interventions.
Keywords: D. melanogaster; ageing; autophagy; cell biology; chromosomes; gene expression; histone; intestine; lifespan; mTOR; mouse.
© 2021, Lu et al.
Conflict of interest statement
YL, JR, JE, LD, TW, JS, OH, RM, SG, LP No competing interests declared
Figures
















Similar articles
-
Sexual identity of enterocytes regulates autophagy to determine intestinal health, lifespan and responses to rapamycin.Nat Aging. 2022 Dec;2(12):1145-1158. doi: 10.1038/s43587-022-00308-7. Epub 2022 Dec 1. Nat Aging. 2022. PMID: 37118538 Free PMC article.
-
The flavonoid 4,4'-dimethoxychalcone promotes autophagy-dependent longevity across species.Nat Commun. 2019 Feb 19;10(1):651. doi: 10.1038/s41467-019-08555-w. Nat Commun. 2019. PMID: 30783116 Free PMC article.
-
MoSnt2-dependent deacetylation of histone H3 mediates MoTor-dependent autophagy and plant infection by the rice blast fungus Magnaporthe oryzae.Autophagy. 2018;14(9):1543-1561. doi: 10.1080/15548627.2018.1458171. Epub 2018 Aug 31. Autophagy. 2018. PMID: 29929416 Free PMC article.
-
The Target of Rapamycin Signalling Pathway in Ageing and Lifespan Regulation.Genes (Basel). 2020 Sep 3;11(9):1043. doi: 10.3390/genes11091043. Genes (Basel). 2020. PMID: 32899412 Free PMC article. Review.
-
Histone variants: key players of chromatin.Cell Tissue Res. 2014 Jun;356(3):457-66. doi: 10.1007/s00441-014-1862-4. Epub 2014 Apr 30. Cell Tissue Res. 2014. PMID: 24781148 Review.
Cited by
-
Genomic Instability and Epigenetic Changes during Aging.Int J Mol Sci. 2023 Sep 19;24(18):14279. doi: 10.3390/ijms241814279. Int J Mol Sci. 2023. PMID: 37762580 Free PMC article. Review.
-
Ageing-associated changes in transcriptional elongation influence longevity.Nature. 2023 Apr;616(7958):814-821. doi: 10.1038/s41586-023-05922-y. Epub 2023 Apr 12. Nature. 2023. PMID: 37046086 Free PMC article.
-
Transcriptional memory of dFOXO activation in youth curtails later-life mortality through chromatin remodeling and Xbp1.Nat Aging. 2022 Dec;2(12):1176-1190. doi: 10.1038/s43587-022-00312-x. Epub 2022 Dec 1. Nat Aging. 2022. PMID: 37118537 Free PMC article.
-
Unraveling Histone Loss in Aging and Senescence.Cells. 2024 Feb 9;13(4):320. doi: 10.3390/cells13040320. Cells. 2024. PMID: 38391933 Free PMC article. Review.
-
ACBP/DBI protein neutralization confers autophagy-dependent organ protection through inhibition of cell loss, inflammation, and fibrosis.Proc Natl Acad Sci U S A. 2022 Oct 11;119(41):e2207344119. doi: 10.1073/pnas.2207344119. Epub 2022 Oct 3. Proc Natl Acad Sci U S A. 2022. PMID: 36191214 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

