Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2
- PMID: 33988688
- PMCID: PMC8129797
- DOI: 10.1182/blood.2021012217
Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2
Abstract
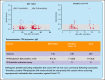
Vaccination using the adenoviral vector COVID-19 vaccine ChAdOx1 nCoV-19 (AstraZeneca) has been associated with rare vaccine-induced immune thrombotic thrombocytopenia (VITT). Affected patients test strongly positive in platelet factor 4 (PF4)/polyanion enzyme immunoassays (EIAs), and serum-induced platelet activation is maximal in the presence of PF4. We determined the frequency of anti-PF4/polyanion antibodies in healthy vaccinees and assessed whether PF4/polyanion EIA+ sera exhibit platelet-activating properties after vaccination with ChAdOx1 nCoV-19 (n = 138) or BNT162b2 (BioNTech/Pfizer; n = 143). In total, 19 of 281 participants tested positive for anti-PF4/polyanion antibodies postvaccination (All: 6.8% [95% confidence interval (CI), 4.4-10.3]; BNT162b2: 5.6% [95% CI, 2.9-10.7]; ChAdOx1 nCoV-19: 8.0% [95% CI, 4.5% to 13.7%]). Optical densities were mostly low (between 0.5 and 1.0 units; reference range, <0.50), and none of the PF4/polyanion EIA+ samples induced platelet activation in the presence of PF4. We conclude that positive PF4/polyanion EIAs can occur after severe acute respiratory syndrome coronavirus 2 vaccination with both messenger RNA- and adenoviral vector-based vaccines, but many of these antibodies likely have minor (if any) clinical relevance. Accordingly, low-titer positive PF4/polyanion EIA results should be interpreted with caution when screening asymptomatic individuals after vaccination against COVID-19. Pathogenic platelet-activating antibodies that cause VITT do not occur commonly following vaccination.
© 2021 by The American Society of Hematology.
Figures

Comment in
-
Understanding VITT(ual) reality.Blood. 2021 Jul 29;138(4):285-286. doi: 10.1182/blood.2021012524. Blood. 2021. PMID: 34323943 Free PMC article. No abstract available.
Similar articles
-
Potential mechanisms of vaccine-induced thrombosis.Eur J Intern Med. 2022 Nov;105:1-7. doi: 10.1016/j.ejim.2022.08.002. Epub 2022 Aug 8. Eur J Intern Med. 2022. PMID: 35953336 Free PMC article. Review.
-
Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination.N Engl J Med. 2021 Jun 3;384(22):2092-2101. doi: 10.1056/NEJMoa2104840. Epub 2021 Apr 9. N Engl J Med. 2021. PMID: 33835769 Free PMC article.
-
A flow cytometric assay to detect platelet-activating antibodies in VITT after ChAdOx1 nCov-19 vaccination.Blood. 2021 Jul 1;137(26):3656-3659. doi: 10.1182/blood.2021012064. Blood. 2021. PMID: 33945605 Free PMC article. Clinical Trial.
-
Low clinical utility of testing for anti-platelet factor 4 in asymptomatic individuals after ChAdOx1 nCoV-19 vaccine.Int J Lab Hematol. 2022 Apr;44(2):424-429. doi: 10.1111/ijlh.13774. Epub 2021 Nov 30. Int J Lab Hematol. 2022. PMID: 34850575
-
The role of anti-platelet factor 4 antibodies and platelet activation tests in patients with vaccine-induced immune thrombotic thrombocytopenia: Brief report on a comparison of the laboratory diagnosis and literature review.Clin Chim Acta. 2022 Apr 1;529:42-45. doi: 10.1016/j.cca.2022.02.003. Epub 2022 Feb 12. Clin Chim Acta. 2022. PMID: 35167842 Free PMC article. Review.
Cited by
-
Potential mechanisms of vaccine-induced thrombosis.Eur J Intern Med. 2022 Nov;105:1-7. doi: 10.1016/j.ejim.2022.08.002. Epub 2022 Aug 8. Eur J Intern Med. 2022. PMID: 35953336 Free PMC article. Review.
-
Prevalence of thrombocytopenia, anti-platelet factor 4 antibodies and D-dimer elevation in Thai people After ChAdOx1 nCoV-19 vaccination.Res Pract Thromb Haemost. 2021 Sep 18;5(6):e12580. doi: 10.1002/rth2.12580. eCollection 2021 Aug. Res Pract Thromb Haemost. 2021. PMID: 34568726 Free PMC article.
-
Heparin-Induced Thrombotic Thrombocytopenia (HITT) and Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT): Similar but Different.Methods Mol Biol. 2023;2663:405-415. doi: 10.1007/978-1-0716-3175-1_26. Methods Mol Biol. 2023. PMID: 37204726
-
Transient Autoreactive PF4 and Antiphospholipid Antibodies in COVID-19 Vaccine Recipients.Vaccines (Basel). 2023 Dec 14;11(12):1851. doi: 10.3390/vaccines11121851. Vaccines (Basel). 2023. PMID: 38140254 Free PMC article.
-
ChAdOx1 vaccination, blood coagulation, and inflammation: No effect on coagulation but increased interleukin-6.Res Pract Thromb Haemost. 2021 Dec 7;5(8):e12630. doi: 10.1002/rth2.12630. eCollection 2021 Dec. Res Pract Thromb Haemost. 2021. PMID: 34934894 Free PMC article.
References
-
- Voysey M, Costa Clemens SA, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881-891. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

