Upregulation of VCAM-1 in lymphatic collectors supports dendritic cell entry and rapid migration to lymph nodes in inflammation
- PMID: 33988714
- PMCID: PMC8129804
- DOI: 10.1084/jem.20201413
Upregulation of VCAM-1 in lymphatic collectors supports dendritic cell entry and rapid migration to lymph nodes in inflammation
Abstract
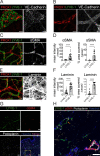
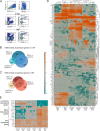
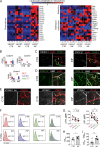
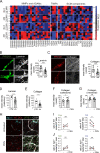
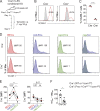
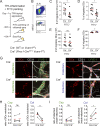
Dendritic cell (DC) migration to draining lymph nodes (dLNs) is a slow process that is believed to begin with DCs approaching and entering into afferent lymphatic capillaries. From capillaries, DCs slowly crawl into lymphatic collectors, where lymph flow induced by collector contraction supports DC detachment and thereafter rapid, passive transport to dLNs. Performing a transcriptomics analysis of dermal endothelial cells, we found that inflammation induces the degradation of the basement membrane (BM) surrounding lymphatic collectors and preferential up-regulation of the DC trafficking molecule VCAM-1 in collectors. In crawl-in experiments performed in ear skin explants, DCs entered collectors in a CCR7- and β1 integrin-dependent manner. In vivo, loss of β1-integrins in DCs or of VCAM-1 in lymphatic collectors had the greatest impact on DC migration to dLNs at early time points when migration kinetics favor the accumulation of rapidly migrating collector DCs rather than slower capillary DCs. Taken together, our findings identify collector entry as a critical mechanism enabling rapid DC migration to dLNs in inflammation.
© 2021 Arasa et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

