Dermatology Life Quality Index in Patients with Moderate-to-Severe Plaque Psoriasis Treated with Brodalumab or Ustekinumab
- PMID: 33988818
- PMCID: PMC8322208
- DOI: 10.1007/s13555-021-00545-5
Dermatology Life Quality Index in Patients with Moderate-to-Severe Plaque Psoriasis Treated with Brodalumab or Ustekinumab
Abstract
Introduction: Targeted biological therapies for psoriasis have resulted in significant benefits, with therapeutic goals such as clear or almost clear skin accompanied by improvements in health-related quality of life (HRQoL). The objective of this study was to compare the effects of 52 weeks of treatment with brodalumab or ustekinumab on HRQoL in patients with moderate-to-severe plaque psoriasis.
Methods: Data were pooled from two randomised controlled phase 3 trials (AMAGINE-2 and -3) which included patients with moderate-to-severe plaque psoriasis treated with brodalumab 210 mg or ustekinumab 45 or 90 mg for 52 weeks. HRQoL outcomes were measured using the Dermatology Life Quality Index (DLQI) as well as the DLQI-Relevant (DLQI-R) version which excludes 'not relevant' responses.
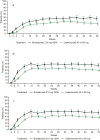
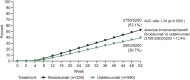
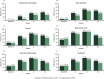
Results: A total of 929 patients were included, 339 in the brodalumab group and 590 in the ustekinumab group. A significantly greater reduction (improvement) in DLQI score from baseline was observed in the brodalumab group compared with the ustekinumab group at weeks 4 [least-squares (LS) mean difference - 2.9, 95% confidence interval [CI] - 3.6 to - 2.2; p < 0.001), 12 (LS mean difference - 0.85, 95% CI - 1.5 to - 0.2; p = 0.01) and 52 (LS mean difference - 0.94, 95% CI - 1.6 to - 0.2; p = 0.009)]. Significantly greater proportions of patients treated with brodalumab achieved a DLQI score of 0 at weeks 4 (15.0 vs. 5.4%; p < 0.0001), 12 (37.5 vs. 28.0%; p = 0.0140) and 52 (46.3 vs. 30.3%; p < 0.0001), or of ≤ 1 [DLQI (0/1): 33.9 vs. 15.4%, 59.9 vs. 45.6% and 54.9 vs. 39.8%, respectively; all p < 0.0001]. Similar results were observed using the DLQI-R scoring system. Significantly more patients achieved a ≥ 4 or ≥ 5 improvement in DLQI with brodalumab compared to ustekinumab at weeks 4 and 52. Treatment with brodalumab was associated with significantly more patients achieving a DLQI of 0 compared to ustekinumab for all domains after 4 and 52 weeks.
Conclusion: Brodalumab was associated with a significantly greater improvement in HRQoL compared to ustekinumab in patients with moderate-to-severe psoriasis.
Keywords: Brodalumab; Pharmacology; Psoriasis; Quality of life; Ustekinumab.
© 2021. The Author(s).
Figures

References
-
- Langley RG, Feldman SR, Han C, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol. 2010;63(3):457–465. doi: 10.1016/j.jaad.2009.09.014. - DOI - PubMed
-
- Papp KA, Gordon KB, Langley RG, et al. Impact of previous biologic use on the efficacy and safety of brodalumab and ustekinumab in patients with moderate-to-severe plaque psoriasis: integrated analysis of the randomized controlled trials AMAGINE-2 and AMAGINE-3. Br J Dermatol. 2018;179:320–328. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

