Potential Use of Exosomes as Diagnostic Biomarkers and in Targeted Drug Delivery: Progress in Clinical and Preclinical Applications
- PMID: 33988964
- PMCID: PMC8147457
- DOI: 10.1021/acsbiomaterials.1c00217
Potential Use of Exosomes as Diagnostic Biomarkers and in Targeted Drug Delivery: Progress in Clinical and Preclinical Applications
Abstract
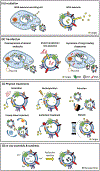
Exosomes are cell-derived vesicles containing heterogeneous active biomolecules such as proteins, lipids, mRNAs, receptors, immune regulatory molecules, and nucleic acids. They typically range in size from 30 to 150 nm in diameter. An exosome's surfaces can be bioengineered with antibodies, fluorescent dye, peptides, and tailored for small molecule and large active biologics. Exosomes have enormous potential as a drug delivery vehicle due to enhanced biocompatibility, excellent payload capability, and reduced immunogenicity compared to alternative polymeric-based carriers. Because of active targeting and specificity, exosomes are capable of delivering their cargo to exosome-recipient cells. Additionally, exosomes can potentially act as early stage disease diagnostic tools as the exosome carries various protein biomarkers associated with a specific disease. In this review, we summarize recent progress on exosome composition, biological characterization, and isolation techniques. Finally, we outline the exosome's clinical applications and preclinical advancement to provide an outlook on the importance of exosomes for use in targeted drug delivery, biomarker study, and vaccine development.
Keywords: Exosome; biomarker; clinical translation; diagnosis; drug delivery; vaccine.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
