Randomized Phase III Trial of Gemcitabine and Cisplatin With Bevacizumab or Placebo in Patients With Advanced Urothelial Carcinoma: Results of CALGB 90601 (Alliance)
- PMID: 33989025
- PMCID: PMC8462587
- DOI: 10.1200/JCO.21.00286
Randomized Phase III Trial of Gemcitabine and Cisplatin With Bevacizumab or Placebo in Patients With Advanced Urothelial Carcinoma: Results of CALGB 90601 (Alliance)
Abstract
Purpose: The combination of gemcitabine and cisplatin (GC) is a standard therapy for metastatic urothelial carcinoma. Based on data that angiogenesis plays a role in urothelial carcinoma growth and progression, a randomized placebo-controlled trial was performed with the primary objective of testing whether patients treated with GC and bevacizumab (GCB) have superior overall survival (OS) than patients treated with GC and placebo (GCP).
Patients and methods: Between July 2009 and December 2014, 506 patients with metastatic urothelial carcinoma without prior chemotherapy for metastatic disease and no neoadjuvant or adjuvant chemotherapy within 12 months were randomly assigned to receive either GCB or GCP. The primary end point was OS, with secondary end points of progression-free survival, objective response, and toxicity.
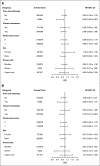
Results: With a median follow-up of 76.3 months among alive patients, the median OS was 14.5 months for patients treated with GCB and 14.3 months for patients treated with GCP (hazard ratio for death = 0.87; 95% CI, 0.72 to 1.05; two-sided stratified log-rank P = .14). The median progression-free survival was 8.0 months for GCB and 6.7 months for GCP (hazard ratio = 0.77; 95% CI, 0.63 to 0.95; P = .016). The proportion of patients with grade 3 or greater adverse events did not differ significantly between both arms, although increased bevacizumab-related toxicities such as hypertension and proteinuria occurred in the bevacizumab-treated arm.
Conclusion: The addition of bevacizumab to GC did not result in improved OS. The observed median OS of about 14 months is consistent with prior phase III trials of cisplatin-based chemotherapy.
Trial registration: ClinicalTrials.gov NCT00942331.
Conflict of interest statement
Figures


Comment in
-
Urological Oncology: Bladder, Penis and Urethral Cancer, and Basic Principles of Oncology.J Urol. 2022 Nov;208(5):1158-1159. doi: 10.1097/JU.0000000000002914. Epub 2022 Aug 19. J Urol. 2022. PMID: 35984094 No abstract available.
References
-
- US Food & Drug Administration FDA approves new, targeted treatment for bladder cancer. https://www.fda.gov/news-events/press-announcements/fda-approves-new-tar...
-
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study J Clin Oncol 183068–30772000 - PubMed
-
- Theodoropoulos VE, Lazaris A, Sofras F, et al. Hypoxia-inducible factor 1 alpha expression correlates with angiogenesis and unfavorable prognosis in bladder cancer Eur Urol 46200–2082004 - PubMed
-
- Theodoropoulos VE, Lazaris AC, Kastriotis I, et al. Evaluation of hypoxia-inducible factor 1alpha overexpression as a predictor of tumour recurrence and progression in superficial urothelial bladder carcinoma BJU Int 95425–4312005 - PubMed
-
- Xia G, Kumar SR, Hawes D, et al. Expression and significance of vascular endothelial growth factor receptor 2 in bladder cancer J Urol 1751245–12522006 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 CA233234/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA233324/CA/NCI NIH HHS/United States
- UG1 CA189858/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- P50 CA221745/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- UG1 CA233327/CA/NCI NIH HHS/United States
- UG1 CA189819/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- UG1 CA233339/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA233331/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

