Guidelines for the measurement of vascular function and structure in isolated arteries and veins
- PMID: 33989082
- PMCID: PMC8321813
- DOI: 10.1152/ajpheart.01021.2020
Guidelines for the measurement of vascular function and structure in isolated arteries and veins
Abstract
The measurement of vascular function in isolated vessels has revealed important insights into the structural, functional, and biomechanical features of the normal and diseased cardiovascular system and has provided a molecular understanding of the cells that constitutes arteries and veins and their interaction. Further, this approach has allowed the discovery of vital pharmacological treatments for cardiovascular diseases. However, the expansion of the vascular physiology field has also brought new concerns over scientific rigor and reproducibility. Therefore, it is appropriate to set guidelines for the best practices of evaluating vascular function in isolated vessels. These guidelines are a comprehensive document detailing the best practices and pitfalls for the assessment of function in large and small arteries and veins. Herein, we bring together experts in the field of vascular physiology with the purpose of developing guidelines for evaluating ex vivo vascular function. By using this document, vascular physiologists will have consistency among methodological approaches, producing more reliable and reproducible results.
Keywords: arteries; contraction; methods; relaxation; veins.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




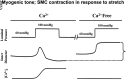







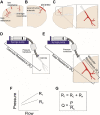

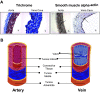







Comment in
-
Reply to De Mey et al.Am J Physiol Heart Circ Physiol. 2022 Apr 1;322(4):H683-H684. doi: 10.1152/ajpheart.00086.2022. Am J Physiol Heart Circ Physiol. 2022. PMID: 35324332 Free PMC article. No abstract available.
-
No guidelines for vascular nerves?Am J Physiol Heart Circ Physiol. 2022 Apr 1;322(4):H681-H682. doi: 10.1152/ajpheart.00053.2022. Am J Physiol Heart Circ Physiol. 2022. PMID: 35324333 Free PMC article. No abstract available.
-
Reply to Boedtkjer and Aalkjaer.Am J Physiol Heart Circ Physiol. 2022 Apr 1;322(4):H687-H688. doi: 10.1152/ajpheart.00117.2022. Am J Physiol Heart Circ Physiol. 2022. PMID: 35324334 Free PMC article. No abstract available.
-
The solution to bicarbonate.Am J Physiol Heart Circ Physiol. 2022 Apr 1;322(4):H685-H686. doi: 10.1152/ajpheart.00057.2022. Am J Physiol Heart Circ Physiol. 2022. PMID: 35324335 Free PMC article. No abstract available.
References
-
- Lubitz SA. Early reactions to Harvey's circulation theory: the impact on medicine. Mt Sinai J Med 71: 4: 274–280, 2004. - PubMed
Publication types
MeSH terms
Grants and funding
- R01HL139585/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- P20 GM130459/GM/NIGMS NIH HHS/United States
- R01HL121871/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- DK115255/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- R61 NS115132/NS/NINDS NIH HHS/United States
- K99HL151889/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01HL151413/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R00HL116769/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01HL091905/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01HL088554/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL139585/HL/NHLBI NIH HHS/United States
- P20GM130459/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- R01 HL135901/HL/NHLBI NIH HHS/United States
- RF1 NS110044/NS/NINDS NIH HHS/United States
- R01ES014639/HHS | NIH | National Institute of Environmental Health Sciences (NIEHS)
- U24 DK076169/DK/NIDDK NIH HHS/United States
- S10OD023438/HHS | NIH | NIH Office of the Director (OD)
- R01HL137112/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01HL135901/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01HL146914/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R00 HL116769/HL/NHLBI NIH HHS/United States
- K99 HL151889/HL/NHLBI NIH HHS/United States
- U24 DK115255/DK/NIDDK NIH HHS/United States
- R21 EB026518/EB/NIBIB NIH HHS/United States
- R01 HL149762/HL/NHLBI NIH HHS/United States
- DK076169/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- R01NS082521/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 HL146054/HL/NHLBI NIH HHS/United States
- R21EB026518/HHS | NIH | National Institute of Biomedical Imaging and Bioengineering (NIBIB)
- R01 HL123301/HL/NHLBI NIH HHS/United States
- P01 HL134604/HL/NHLBI NIH HHS/United States
- R00GM118885/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- R01 HL091905/HL/NHLBI NIH HHS/United States
- RF1NS110044/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01HL142808/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R61NS115132/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01HL146562/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL088105/HL/NHLBI NIH HHS/United States
- SB1 HL121871/HL/NHLBI NIH HHS/United States
- R01 HD037831/HD/NICHD NIH HHS/United States
- R01 HL137852/HL/NHLBI NIH HHS/United States
- R35 HL155008/HL/NHLBI NIH HHS/United States
- R01 HL137112/HL/NHLBI NIH HHS/United States
- R01HL149762/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01HL123301/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL146914/HL/NHLBI NIH HHS/United States
- R01 HL142808/HL/NHLBI NIH HHS/United States
- R01 HL088554/HL/NHLBI NIH HHS/United States
- R01HD037831/HHS | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- R01HL146054/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL146562/HL/NHLBI NIH HHS/United States
- R44 HL121871/HL/NHLBI NIH HHS/United States
- R01HL088105/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 ES014639/ES/NIEHS NIH HHS/United States
- P01HL134604/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01HL137852/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- S10 OD023438/OD/NIH HHS/United States
- R01 HL151413/HL/NHLBI NIH HHS/United States
- R41 HL121871/HL/NHLBI NIH HHS/United States
- R00 GM118885/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

