Is the HPV-test more cost-effective than cytology in cervical cancer screening? An economic analysis from a middle-income country
- PMID: 33989331
- PMCID: PMC8121350
- DOI: 10.1371/journal.pone.0251688
Is the HPV-test more cost-effective than cytology in cervical cancer screening? An economic analysis from a middle-income country
Abstract
Objective: To report a modelling study using local health care costs and epidemiological inputs from a population-based program to access the cost-effectiveness of adopting hrHPV test.
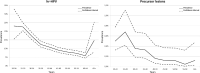
Methods: A cost-effectiveness analysis based on a microsimulation dynamic Markov model. Data and costs were based on data from the local setting and literature review. The setting was Indaiatuba, Brazil, that has adopted the hrHPV test in place of cytology since 2017. After calibrating the model, one million women were simulated in hypothetical cohorts. Three strategies were tested: cytology to women aged 25 to 64 every three years; hrHPV test to women 25-64 every five years; cytology to women 25-29 years every three years and hrHPV test to women 30-64 every five years (hybrid strategy). Outcomes were Quality-adjusted life-years (QALY) and Incremental Cost-Effectiveness Ratio (ICER).
Results: The hrHPV testing and the hybrid strategy were the dominant strategies. Costs were lower and provided a more effective option at a negative incremental ratio of US$ 37.87 for the hybrid strategy, and negative US$ 6.16 for the HPV strategy per QALY gained. Reduction on treatment costs would influence a decrease in ICER, and an increase in the costs of the hrHPV test would increase ICER.
Conclusions: Using population-based data, the switch from cytology to hrHPV testing in the cervical cancer screening program of Indaiatuba is less costly and cost-effective than the old cytology program.
Conflict of interest statement
This project is funded by UNICAMP (Women’s Hospital), Indaiatuba City (SUS), and Roche Diagnostics®, as detailed below: This study proposal (screening program implementation and cost-effectiveness analyses) was designed by researchers from UNICAMP and introduced by the Indaiatuba City Hall. Both UNICAMP and the municipality use the existing and functioning structure to place the new screening program and carry out the proposed study at no additional cost. The supplies and equipment required to perform HPV testing (for one round in five years), computer system development support, two lab technicians, and a screening program coordinator are provided or supported by Roche Diagnostics®. Roche Diagnostics® supports an external statistician defined by researchers to develop a model and perform the planned cost-effectiveness analyses. There are no planned compensations or cash transfers provided to any institution or researchers stated in the parties’ cooperation agreement. Roche Diagnostics® support does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Arbyn et al., editors. European Guidelines for Quality Assurance in Cervical Cancer Screening. Second edition. Luxembourg: Office for Official Publications of the European Communities; 2008.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

