The role of TNFRSF11B in development of osteoarthritic cartilage
- PMID: 33989379
- PMCID: PMC8824428
- DOI: 10.1093/rheumatology/keab440
The role of TNFRSF11B in development of osteoarthritic cartilage
Abstract
Objectives: OA is a complex genetic disease with different risk factors contributing to its development. One of the genes, TNFRSF11B, previously identified with gain-of-function mutation in a family with early-onset OA with chondrocalcinosis, is among the highest upregulated genes in lesioned OA cartilage (RAAK-study). Here, we determined the role of TNFRSF11B overexpression in development of OA.
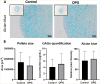
Methods: Human primary articular chondrocytes (9 donors RAAK study) were transduced using lentiviral particles with or without TNFRSF11B. Cells were cultured for 1 week in a 3 D in-vitro chondrogenic model. TNFRSF11B overexpression was confirmed by RT-qPCR, immunohistochemistry and ELISA. Effects of TNFRSF11B overexpression on cartilage matrix deposition, matrix mineralization, and genes highly correlated to TNFRSF11B in RNA-sequencing dataset (r >0.75) were determined by RT-qPCR. Additionally, glycosaminoglycans and collagen deposition were visualized with Alcian blue staining and immunohistochemistry (COL1 and COL2).
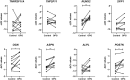
Results: Overexpression of TNFRSF11B resulted in strong upregulation of MMP13, COL2A1 and COL1A1. Likewise, mineralization and osteoblast characteristic markers RUNX2, ASPN and OGN showed a consistent increase. Among 30 genes highly correlated to TNFRSF11B, expression of only eight changed significantly, with BMP6 showing the highest increase (9-fold) while expression of RANK and RANKL remained unchanged indicating previously unknown downstream pathways of TNFRSF11B in cartilage.
Conclusion: Results of our 3D in vitro chondrogenesis model indicate that upregulation of TNFRSF11B in lesioned OA cartilage may act as a direct driving factor for chondrocyte to osteoblast transition observed in OA pathophysiology. This transition does not appear to act via the OPG/RANK/RANKL triad common in bone remodeling.
Keywords: TNFRSF11B; CCAL1; cartilage hypertrophy; chondrogenesis; matrix mineralization; osteoarthritis.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

