Nanopore sensing: A physical-chemical approach
- PMID: 33989531
- PMCID: PMC9793329
- DOI: 10.1016/j.bbamem.2021.183644
Nanopore sensing: A physical-chemical approach
Abstract
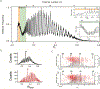
Protein nanopores have emerged as an important class of sensors for the understanding of biophysical processes, such as molecular transport across membranes, and for the detection and characterization of biopolymers. Here, we trace the development of these sensors from the Coulter counter and squid axon studies to the modern applications including exquisite detection of small volume changes and molecular reactions at the single molecule (or reactant) scale. This review focuses on the chemistry of biological pores, and how that influences the physical chemistry of molecular detection.
Keywords: DNA sequencing; Ion channel; Nanopore sensor; Peptide detection; Porin.
Published by Elsevier B.V.
Figures





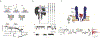
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

