Sodium Butyrate Supplementation Inhibits Hepatic Steatosis by Stimulating Liver Kinase B1 and Insulin-Induced Gene
- PMID: 33989817
- PMCID: PMC8346675
- DOI: 10.1016/j.jcmgh.2021.05.006
Sodium Butyrate Supplementation Inhibits Hepatic Steatosis by Stimulating Liver Kinase B1 and Insulin-Induced Gene
Abstract
Background and aims: Butyric acid is an intestinal microbiota-produced short-chain fatty acid, which exerts salutary effects on alleviating nonalcoholic fatty liver disease (NAFLD). However, the underlying mechanism of butyrate on regulating hepatic lipid metabolism is largely unexplored.
Methods: A mouse model of NAFLD was induced with high-fat diet feeding, and sodium butyrate (NaB) intervention was initiated at the eighth week and lasted for 8 weeks. Hepatic steatosis was evaluated and metabolic pathways concerning lipid homeostasis were analyzed.
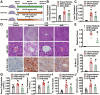
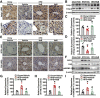
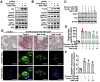
Results: Here, we report that administration of NaB by gavage once daily for 8 weeks causes an augmentation of insulin-induced gene (Insig) activity and inhibition of lipogenic gene in mice fed with high-fat diet. Mechanistically, NaB is sufficient to enhance the interaction between Insig and its upstream kinase AMP-activated protein kinase (AMPK). The stimulatory effects of NaB on Insig-1 activity are abolished in AMPKα1/α2 double knockout (AMPK-/-) mouse primary hepatocytes. Moreover, AMPK activation by NaB is mediated by LKB1, as evidenced by the observations showing NaB-mediated induction of phosphorylation of AMPK, and its downstream target acetyl-CoA carboxylase is diminished in LKB1-/- mouse embryonic fibroblasts.
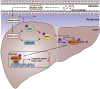
Conclusions: These studies indicate that NaB serves as a negative regulator of hepatic lipogenesis in NAFLD and that NaB attenuates hepatic steatosis and improves lipid profile and liver function largely through the activation of LKB1-AMPK-Insig signaling pathway. Therefore, NaB has therapeutic potential for treating NAFLD and related metabolic diseases.
Keywords: Hepatic Lipogenesis; Insulin-Induced Gene; LKB1; NAFLD; Sodium Butyrate.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Fan J.G., Kim S.U., Wong V.W. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–873. - PubMed
-
- Paik J.M., Golabi P., Younossi Y., Mishra A., Younossi Z.M. Changes in the global burden of chronic liver diseases from 2012 to 2017: The growing impact of nonalcoholic fatty liver disease. Hepatology. 2020;72:1605–1616. - PubMed
-
- Smith G.I., Shankaran M., Yoshino M., Schweitzer G.G., Chondronikola M., Beals J.W., Okunade A.L., Patterson B.W., Nyangau E., Field T., Sirlin C.B., Talukdar S., Hellerstein M.K., Klein S. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 2020;130:1453–1460. - PMC - PubMed
-
- Goedeke L., Bates J., Vatner D.F., Perry R.J., Wang T., Ramirez R., Li L., Ellis M.W., Zhang D., Wong K.E., Beysen C., Cline G.W., Ray A.S., Shulman G.I. Acetyl-CoA carboxylase inhibition reverses nafld and hepatic insulin resistance but promotes hypertriglyceridemia in rodents. Hepatology. 2018;68:2197–2211. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

