MASSIVE ADVANCING NONEXUDATIVE TYPE 1 CHOROIDAL NEOVASCULARIZATION IN CTRP5 LATE-ONSET RETINAL DEGENERATION: Longitudinal Findings on Multimodal Imaging and Implications for Age-Related Macular Degeneration
- PMID: 33990119
- PMCID: PMC8542642
- DOI: 10.1097/IAE.0000000000003205
MASSIVE ADVANCING NONEXUDATIVE TYPE 1 CHOROIDAL NEOVASCULARIZATION IN CTRP5 LATE-ONSET RETINAL DEGENERATION: Longitudinal Findings on Multimodal Imaging and Implications for Age-Related Macular Degeneration
Abstract
Purpose: To describe longitudinal multimodal imaging findings of nonexudative choroidal neovascularization in CTRP5 late-onset retinal degeneration.
Methods: Four patients with CTRP5-positive late-onset retinal degeneration underwent repeated ophthalmoscopic examination and multimodal imaging. All four patients (two siblings and their cousins, from a pedigree described previously) had the heterozygous S163R mutation.
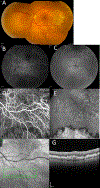
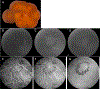
Results: All four patients demonstrated large subretinal lesions in the mid-peripheral retina of both eyes. The lesions were characterized by confluent hypercyanescence with hypocyanescent borders on indocyanine green angiography, faintly visible branching vascular networks with absent/minimal leakage on fluorescein angiography, Type 1 neovascularization on optical coherence tomography angiography, and absent retinal fluid, consistent with nonexudative choroidal neovascularization. The neovascular membranes enlarged substantially over time and the birth of new membranes was observed, but all lesions remained nonexudative/minimally exudative. Without treatment, all involved retinal areas remained free of atrophy and subretinal fibrosis.
Conclusion: We report the existence of massive advancing nonexudative Type 1 choroidal neovascularization in CTRP5 late-onset retinal degeneration. These findings have implications for age-related macular degeneration. They provide a monogenic model system for studying the mechanisms underlying the distinct events of choroidal neovascularization development, enlargement, progression to exudation, and atrophy in age-related macular degeneration. They suggest that choroidal hypoperfusion precedes neovascularization and that nonexudative neovascularization may protect against atrophy.
Conflict of interest statement
Figures





Similar articles
-
Multimodal imaging of late-onset retinal degeneration complicated by bilateral choroidal neovascularization.Eye (Lond). 2019 Jun;33(6):1020-1027. doi: 10.1038/s41433-019-0348-8. Epub 2019 Jan 28. Eye (Lond). 2019. PMID: 30692649 Free PMC article. No abstract available.
-
Prevalence and Risk Factors for Nonexudative Neovascularization in Fellow Eyes of Patients With Unilateral Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy.Invest Ophthalmol Vis Sci. 2017 Jul 1;58(9):3488-3495. doi: 10.1167/iovs.16-21167. Invest Ophthalmol Vis Sci. 2017. PMID: 28702676
-
Incidence of Fellow Eye Involvement in Patients With Unilateral Exudative Age-Related Macular Degeneration.JAMA Ophthalmol. 2018 Aug 1;136(8):905-911. doi: 10.1001/jamaophthalmol.2018.2154. JAMA Ophthalmol. 2018. PMID: 29879284 Free PMC article.
-
Optical coherence tomography angiography in age-related macular degeneration: The game changer.Eur J Ophthalmol. 2018 Jul;28(4):349-357. doi: 10.1177/1120672118766807. Epub 2018 Apr 6. Eur J Ophthalmol. 2018. PMID: 29623720 Review.
-
Nonexudative Macular Neovascularization - A Systematic Review of Prevalence, Natural History, and Recent Insights from OCT Angiography.Ophthalmol Retina. 2020 Jul;4(7):651-661. doi: 10.1016/j.oret.2020.02.016. Epub 2020 Mar 13. Ophthalmol Retina. 2020. PMID: 32335033 Free PMC article.
Cited by
-
Outer retinal corrugations in late-onset retinal degeneration: a diagnostic finding demonstrated with multimodal imaging.BMJ Open Ophthalmol. 2023 Oct;8(1):e001370. doi: 10.1136/bmjophth-2023-001370. BMJ Open Ophthalmol. 2023. PMID: 37884319 Free PMC article.
-
Late-Onset Retinal Degeneration: Clinical Perspectives.Clin Ophthalmol. 2022 Sep 30;16:3225-3246. doi: 10.2147/OPTH.S362691. eCollection 2022. Clin Ophthalmol. 2022. PMID: 36204011 Free PMC article. Review.
-
Longitudinal Changes of Retinal Structure in Molecularly Confirmed C1QTNF5 Patients With Late-Onset Retinal Degeneration.Transl Vis Sci Technol. 2023 Dec 1;12(12):14. doi: 10.1167/tvst.12.12.14. Transl Vis Sci Technol. 2023. PMID: 38085246 Free PMC article.
References
-
- Kuntz CA, Jacobson SG, Cideciyan AV, et al. Sub-retinal pigment epithelial deposits in a dominant late-onset retinal degeneration. Invest Ophthalmol Vis Sci. 1996;37(9):1772–1782. - PubMed
-
- Ayyagari R, Griesinger IB, Bingham E, et al. Autosomal dominant hemorrhagic macular dystrophy not associated with the TIMP3 gene. Arch Ophthalmol. 2000;118(1):85–92. - PubMed
-
- Milam AH, Curcio CA, Cideciyan AV, et al. Dominant late-onset retinal degeneration with regional variation of sub-retinal pigment epithelium deposits, retinal function, and photoreceptor degeneration. Ophthalmology. 2000;107(12):2256–2266. - PubMed
-
- Jacobson SG, Cideciyan AV, Wright E, Wright AF. Phenotypic marker for early disease detection in dominant late-onset retinal degeneration. Invest Ophthalmol Vis Sci. 2001;42(8):1882–1890. - PubMed
-
- Hayward C, Shu X, Cideciyan AV, et al. Mutation in a short-chain collagen gene, CTRP5, results in extracellular deposit formation in late-onset retinal degeneration: a genetic model for age-related macular degeneration. Hum Mol Genet. 2003;12(20):2657–2667. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

