Impact of concomitant medication on clinical outcomes in patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors: A retrospective study
- PMID: 33990133
- PMCID: PMC8258365
- DOI: 10.1111/1759-7714.14001
Impact of concomitant medication on clinical outcomes in patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors: A retrospective study
Abstract
Background: It has recently been suggested that concomitant medication may affect the clinical outcome of patients treated with immune checkpoint inhibitors (ICIs). However, only a few studies on the impact of concomitant medication on immune-related adverse events (irAEs) have previously been reported. Here, we aimed to determine the impact of concomitant medication on the efficacy and safety of ICIs.
Methods: We retrospectively analyzed the data of 300 patients treated with nivolumab or pembrolizumab for advanced non-small cell lung cancer (NSCLC) between January 2016 and July 2018. Multivariate logistic regression analysis was used to assess the effect of concomitant medication on treatment response or irAEs. A multivariate Cox proportional hazards model was used to evaluate concomitant medication-related factors associated with time-to-treatment failure or overall survival (OS).
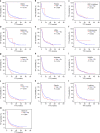
Results: A total of 70 patients responded to treatment and 137 experienced irAEs. The response rate and incidence of irAEs in patients treated with ICIs were not significantly associated with concomitant medication. Multivariate analysis showed that the use of opioids was an independent factor (time-to-treatment failure: hazard ratio 1.39, p = 0.021, OS: hazard ratio 1.54, p = 0.007).
Conclusions: The efficacy and safety of nivolumab or pembrolizumab in the treatment of patients with advanced NSCLC were not significantly influenced by concomitant medication. However, opioid usage might be associated with shorter OS in patients treated with these ICIs. Further mechanistic investigations should explore whether these associations are purely prognostic or contribute to ICI resistance.
Keywords: concomitant medication; immune checkpoint inhibitors; immune-related adverse events; non-small cell lung cancer; opioids.
© 2021 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. - PubMed
-
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, ET MF, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–55. - PubMed
-
- Califano R, Lal R, Lewanski C, Nicolson MC, Ottensmeier CH, Popat S, et al. Patient selection for anti‐PD‐1/PD‐L1 therapy in advanced non‐small cell lung cancer: implications for clinical practice. Future Oncol. 2018;14:2415–31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

